Introduction: Annulus fibrosus (AF) injuries commonly lead to substantial intervertebral disc (IVD) degeneration, the major cause of lower back pain which affects about 80% of the population. Recently, tissue engineering has evolved into a promising approach for AF regeneration. While a lot of attempts have been made during the last decade, constructing engineered AFs remains challenging due to the tremendous complexity of AF tissue at cellular, biochemical, microstructural, and biomechanical levels. It is known that the elasticity of matrix effectively directs the lineage specification of stem cells. In this study, we synthesized a series of biodegradable poly(ether carbonate urethane)urea (PECUU) materials whose elasticity approximated that of native AF tissue. We then explored the potential of AF-derived stem cells (AFSCs) to achieve diversified differentiation of cells by varying the elasticity of substrate. We found that the elasticity of scaffold was likely a determinant factor in AF tissue engineering.
Materials and Methods: A series of biodegradable PECUUs with tunable elastic modulus were synthesized by a one-pot, three-step polymerization. Fibrous PECUU scaffolds were fabricated by electrospinning technique and used for culturing AFSCs. The growth, gene expression, biochemical and biomechanical characteristics of AFSCs were studied.
Results and Discussion: By adjusting the molecular weight of polycarbonates, ratios of hard segment to soft segment, a series of polyurethanes were obtained with different elastic modulus (PECUU1, 13.4MPa; PECUU2, 6.4MPa; PECUU3, 5.1MPa; PECUU4, 2.5MPa), which is close to the elastic modulus of AF tissue. When AFSCs were cultured on electrospun PECUU fibrous scaffolds, the gene expression of collagen-I in them increased with the elasticity of scaffold material, whereas the expression of collagen-II and aggrecan genes showed an opposite trend (Figure 1A-C). At protein level, the content of collagen-I gradually increased with substrate elasticity, while collagen-II and GAG contents decreased (Figure 1D-F). In addition, the cell traction forces (CTFs) of AFSCs gradually decreased with scaffold elasticity (Figure 2). Such substrate elasticity-dependent changes of AFSCs were similar to the gradual transition in the gene, biochemical, and biomechanical characteristics of cells from inner to outer regions of native AF tissue.
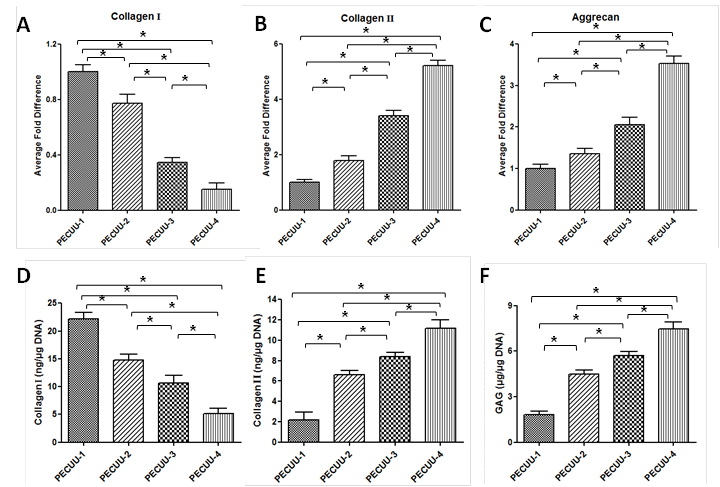
Figure 1. (A-C) Expression of collagen-I, collagen-II, and aggrecan genes in AFSCs cultured on various PECUU fibrous scaffolds for 7 days. (D-F) Biochemical analyses of AFSCs cultured on various PECUU fibrous scaffolds for 7 days.
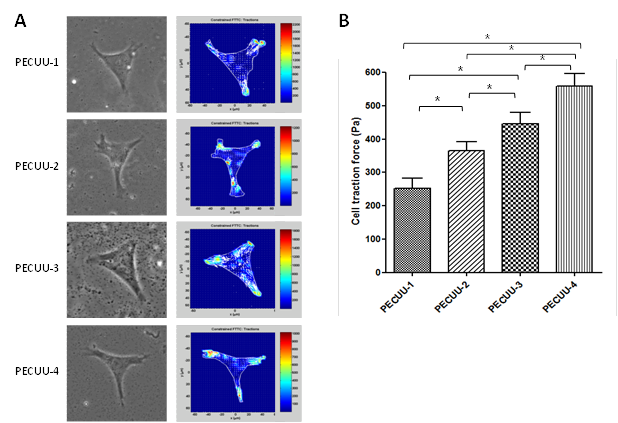
Figure 2. CTFM analysis of AFSCs cultured on various PECUU fibrous scaffolds for 7 days. (A) CTF maps showing the distribution of CTFs throughout a single cell; (B) CTFs of cells on various scaffolds.
Conclusion: Together, findings from this study have, for the first time, implied that AFSCs, depending on the substrate elasticity, had strong tendency to differentiate into various types of AF-like cells. Therefore, this study provides solid basis for the use of AFSCs, along with scaffolds of varying elasticity, for AF tissue engineering.
National Natural Science Foundation of China (81171479, 51303120, 81471790); China Postdoctoral Science Foundation (2012M521121); Natural Science Foundation of Jiangsu Province (BK20130335); Jiangsu Provincial Special Program of Medical Science (BL2012004)