Introduction: After the implantation of a medical device, adhesive proteins such as fibronectin (FN) are adsorbed to the surface of the material[1]. These are the biomolecules that monocytes (MCs) will interact with, to adopt a pro-inflammatory or pro-wound healing phenotype[2]. Hence, it was desired to study FN coatings on two surface modified polyetherurethanes (PEUs) with potential blood-contacting applications, to mimic the biological environment. A fluorinated surface modifying oligomer (PPO212L), known to reduce platelet adhesion[3] and an anionic/dihydroxy oligomeric (ADO) additive, known to enable cell adhesion[4] were used to modify surface blood cell compatibility of a commercial PEU. FN adsorption and the subsequent MC inflammatory response was investigated on the two modified PEUs.
Materials and Methods: The PEU was prepared by dissolving Tecoflex® 65D in 10 % (wt/v) chloroform. PPO212L or ADO was added at 0.5 % (wt/wt). Solutions were casted on PTFE for 24 h followed by 2 h in vacuum (30 mmHg), both at room temperature. Protein adsorption was performed with a FN solution (50 μg.mL-1 in HEPES 10 mM, pH 7.4) at 37 °C for 30 min. Surfaces were characterized by water contact angle (WCA), atomic force microscopy (AFM) and immunofluorescence staining (IFS). Adsorbed FN was quantified by a bicinchoninic acid assay. MCs, isolated from blood of healthy donors (University of Toronto ethics protocol #22203) were seeded on PEUs (106 cells.mL-1). After 72 h of culture, the viability of adhered MCs was evaluated by Alamar blue® assay and cell morphology was analyzed by scanning electron microscopy (SEM). Results were given as mean ± standard deviation (SD) and were statistically analyzed by one-way analysis of variance (ANOVA), with significant differences reported for p<0.05.
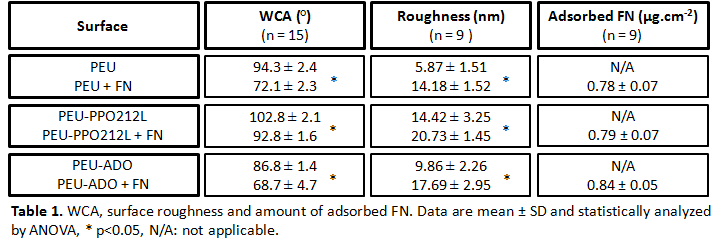
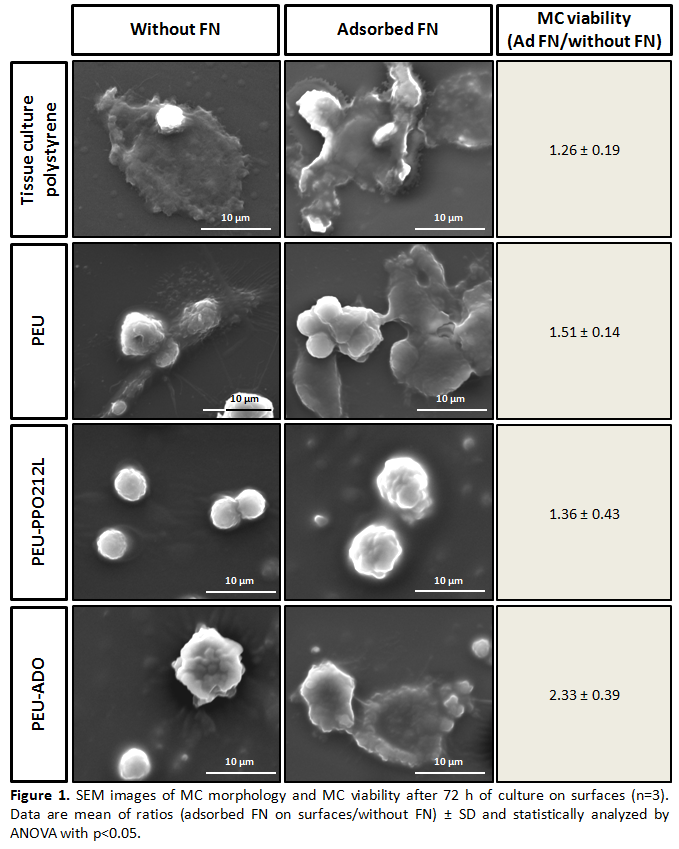
Results and Discussion: On all PEUs, FN adsorption significantly decreased the WCA whereas surface roughness increased approximately 2 fold (Table 1). No statistically significant difference was observed for the amount of adsorbed FN (Table 1) corroborating the presence of a protein monolayer to surfaces[5]. The difference in WCA prior vs after FN adsorption was greatest for the base PEU and PEU-ADO. This result suggested a different FN conformation on PEU-PPO212L than on the two other surfaces. The availability of FN cell binding domains for all coated PEUs was observed after IFS (data not shown). Preliminary tests with MCs showed that all FN-coated PEUs significantly increased MC viability, without affecting cell morphology except for PEU-ADO (Figure 1). These results suggested that FN activated MCs more extensively on PEU-ADO than on the base PEU and PEU-PPO212L, which showed the least activated morphology of all surfaces.
Conclusions: A comparative study of two surface modified PEUs, PEU-PPO212L and PEU-ADO, intended for blood-contacting applications was performed. Investigations suggested that FN-coated PEU-PPO212L has less influence on MC activation than FN-coated PEU-ADO. Further investigations are ongoing to determine the effect of FN coating on cell production of cytokines such as pro-inflammatory tumor necrosis factor-α (TNF-α) and anti-inflammatory interleukin 10 (IL-10).
This work was supported by a MSc Scholarship from NSERC CREATE Program in Regenerative Medicine (www.ncprm.ulaval.ca), NSERC, CIHR, Fonds Samuel de Champlain, FQRS, and FQRNT; The authors would like to thank Pascale Chevallier and Lucie Levesque for their technical assistance
References:
[1] T. a. Horbett, “Chapter 13 Principles underlying the role of adsorbed plasma proteins in blood interactions with foreign materials,” Cardiovasc. Pathol., vol. 2, no. 3, pp. 137–148, Sept. 1993
[2] K. G. Battiston, R. S. Labow, and J. P. Santerre, “Protein binding mediation of biomaterial-dependent monocyte activation on a degradable polar hydrophobic ionic polyurethane,” Biomaterials, vol. 33, no. 33, pp. 8316–8328, Aug. 2012
[3] A. R. Jahangir, W. G. McClung, R. M. Cornelius, C. B. McCloskey, J. L. Brash, and J. P. Santerre, “Fluorinated surface-modifying macromolecules: modulating adhesive protein and platelet interactions on a polyether-urethane.,” J. Biomed. Mater. Res., vol. 60, no. 1, pp. 135–47, Apr. 2002
[4] L. Yang, R. a Kandel, G. Chang, and J. P. Santerre, “Polar surface chemistry of nanofibrous polyurethane scaffold affects annulus fibrosus cell attachment and early matrix accumulation.,” J. Biomed. Mater. Res. A, vol. 91, no. 4, pp. 1089–99, Dec. 2008
[5] L. Baujard-Lamotte, S. Noinville, F. Goubard, P. Marque, and E. Pauthe, “Kinetics of conformational changes of fibronectin adsorbed onto model surfaces,” Colloids Surfaces B Biointerfaces, vol. 63, no. 1, pp. 129–137, May 2008