Introduction
Controlled-release systems that respond to external stimuli have received great interest in the use of medical treatments such as for drug delivery to the specific sites. Previously, we constructed a controlled-release system by combining a cycloadduct of the Diels-Alder reaction and the gold nanorods, that is, we modified gold nanorods with polyethylene glycol (PEG) through Diels-Alder cycloadducts.[1] When the modified gold nanorods were irradiated by near-infrared light, the PEG chains were released from the gold nanorods due to the retro Diels-Alder reaction induced by the photothermal effect.
Dendritic poly(L-lysine) is a mono-dispersed dendritic molecule consisting of L-lysines.[2] PEG-modified dendritic poly(L-lysine) could stably circulate in the blood stream, and accumulate in tumors by the enhanced permeability and retention (EPR) effect. Recently, the dendritic poly (L-lysine) was modified with polyethylene glycol (PEG)-linked hydrophobic peptides. The hydrophobic cavity was formed between the KG6 and PEG chains and then doxorubicin (DOX) was encapsulated in this cavity.[3]
In this study, we combined the controlled-release system using the Diels-Alder cycloadduct with drug delivery system mediated by the dendritic poly(L-lysine). It enables to release drugs in response to heat treatment.
Results and Discussion
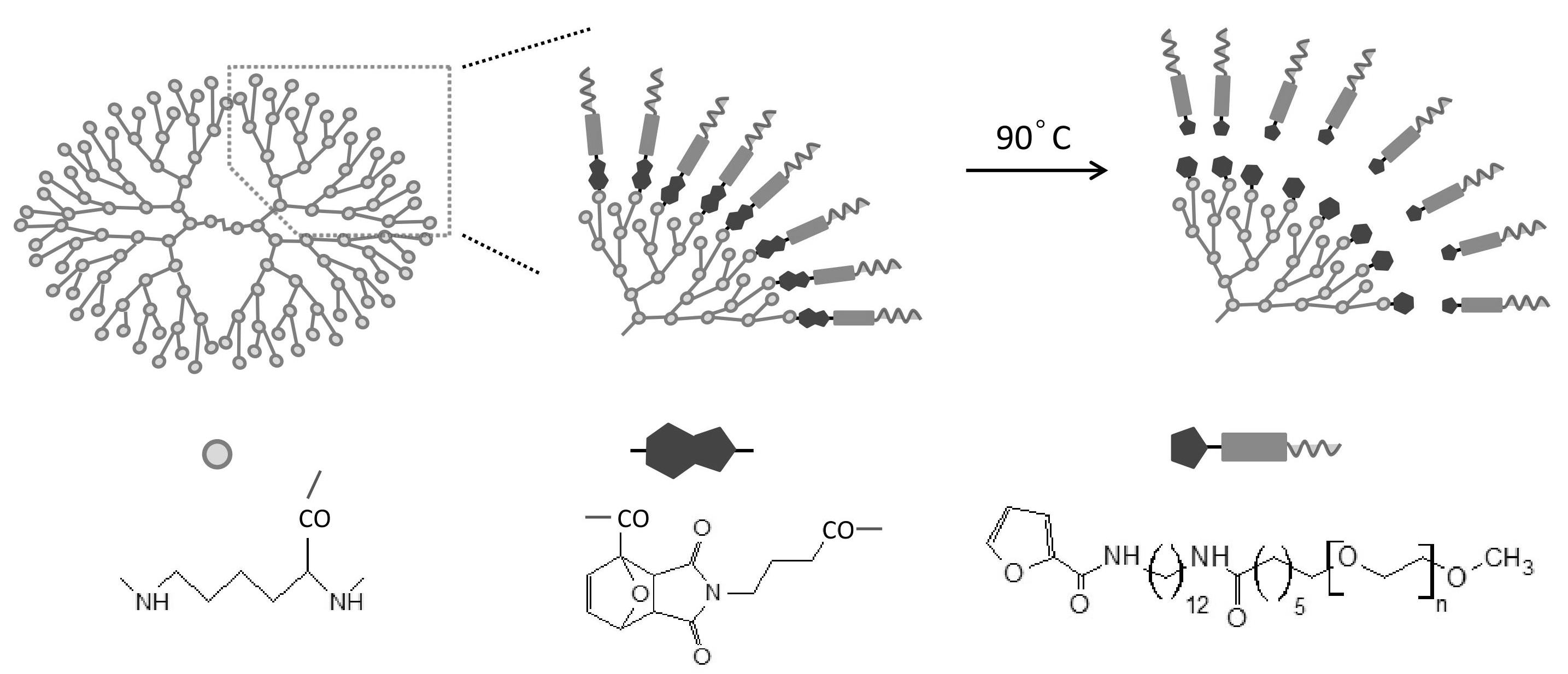
Dendritic poly(L-lysine) was synthesized as previously described.[2] PEG5000-NHS was reacted with an amine group of 1,12-dodecanediamine (C12), then 2-furancarboxylic acid was coupled with another amine group. Resultant PEG-C12-furan was mixed with 4-maleimidobutyric acid,and heated at 60ºC for 3 days. Diels-Alder reaction between the maleimide and the furan groups proceeded. PEG-C12-cycloadduct-COOH was modified terminal amine groups of dendritic poly (L-lysine).
8-anilino-1-naphthalene-sulfonic acid (ANS) was used as a probe for the formation of the hydrophobic cavity on the dendritic poly (L-lysine). After mixing ANS with the dendritic poly (L-lysine), the high fluorescence intensity was observed. When the solution was heated at 90ºC for 10 min, the fluorescence intensity decreased gradually. Since control molecules that have succinyl group instead of the cycloadduct, there was no decrease in the fluorescence intensity while it was heating. It indicated that PEG-C12-furan was released by retroDiels-Alder reaction and the hydrophobic cavity was broken. As a result, ANS was released to the aqueous solution.
Next, we mixed gold nanorods to the system. The gold nanorods have strong absorption at near infrared light region and show photothermal effect, i.e., the gold nanorods can be heated by near-infrared light irradiation. When the solution including the gold nanorods and ANS-encapsulated PEG-C12-cycloadduct-dendritic poly(L-lysine) was irradiated by near-infrared light, it was observed the significant decrease of fluorescence intensity of ANS.
Conclusion
We constructed ANS release system that could be controlled with near-infrared light irradiation by combining the retro Diels-Alder reaction and the photothermal effect of gold nanorods. It might be able to apply in actual drug release system controlled by light irradiation.
This research was supported by a promotion program of the NOVARATIS Foundation (Japan), and a Research grants of GSST Research Core and Group for Research B of Kumamoto University.
References:
[1] S. Yamashita, H. Fukushima, Y. Niidome, T. Mori, Y. Katayama, T. Niidome, Controlled-release system mediated by a retro Diels-Alder reaction induced by the photothermal effect of gold nanorods, Langmuir 27, 14621-14626 (2011)
[2] M. Ohsaki, T. Okuda, A. Wada, T. Hirayama, T. Niidome, H. Aoyagi, In Vitro Gene Transfection Using Dendritic Poly(L-Lysine), Bioconjugate Chem., 13, 510-517 (2002)
[3] T. Niidome, H. Yamauchi, K. Takahashi, K. Naoyama, K. Watanabe, T. Mori, Y. Katayama, Hydrophobic cavity formed by oligopeptide for doxorubicin delivery based on dendritic poly(L-lysine), J. Biomater. Sci. Polym. Ed., 25, 1362-1373 (2014)