Decoration of RGD-mimetic porous scaffold with engineered, devitalized adipose matrix
Eleonora
Rossi1, 2, 3, 4,
Alessandro
Tocchio2, 3, 5,
Irini
Gerges3, 5,
Arnaud
Scherberich1, 4,
Cristina
Lenardi3, 6 and
Ivan
Martin1, 4
-
1
University Hospital, Institute for Surgical Research and Hospital Management, ICFS, Switzerland
-
2
European School of Molecular Medicine, SEMM, Italy
-
3
Filarete Foundation, Advanced Biomaterials, Italy
-
4
University Hospital, Department of Biomedicine, Switzerland
-
5
TENSIVE S. r. l, Italy
-
6
CIMaINa, University of Milan, Physics, Italy
Introduction: Numerous scaffolding materials based on either synthetic or natural polymers have been investigated in the context of adipose tissue engineering for regenerative medicine applications. In parallel, devitalized native adipose tissue has shown great in vivo potential to enhance regeneration and instruct repair of damaged soft tissues. Our ultimate goal is thus to combine a 3D scaffold made of a synthetic polymer, designed to match the mechanical properties of native adipose tissue, with an engineered, devitalized Extracellular Matrix (ECM), providing the biochemical signals of fat tissue. Our working hypothesis is that the combination of these two components provides an adipo-inductive material to be used for fat tissue enhancement in plastic and reconstructive surgery. Towards this goal, here we aimed at developing an RGD-mimetic poly(amidoamine) oligomer foam (OPAAF), and at testing the feasibility of decorating it with ECM, deposited by human Adipose Stromal Cells (hASCs) in vitro and subsequently devitalized.
Materials and Methods: RGD-mimetic poly(amidoamine) oligomers were cross-linked by free radical polymerization. Scanning Electron Microscopy (SEM) analysis was performed to assess pore size. Mechanical properties were evaluated by Dynamic Mechanical Analysis (DMA). In vivo, OPAAF’s biocompatibility was assessed through subcutaneous implantation in a mouse model. In vitro, a perfusion bioreactor system was used to uniformly seed and culture hASCs with adipogenic differentiation medium. Constructs were then analyzed by BODIPY staining for lipid droplets and RT-PCR analysis for PPARg and FABP4. Freeze and Thaw (F/T) cycles were used to devitalize the constructs and to generate a hybrid ECM-OPAAF. The presence of collagen IV in ECM was assessed by immunofluorescence.
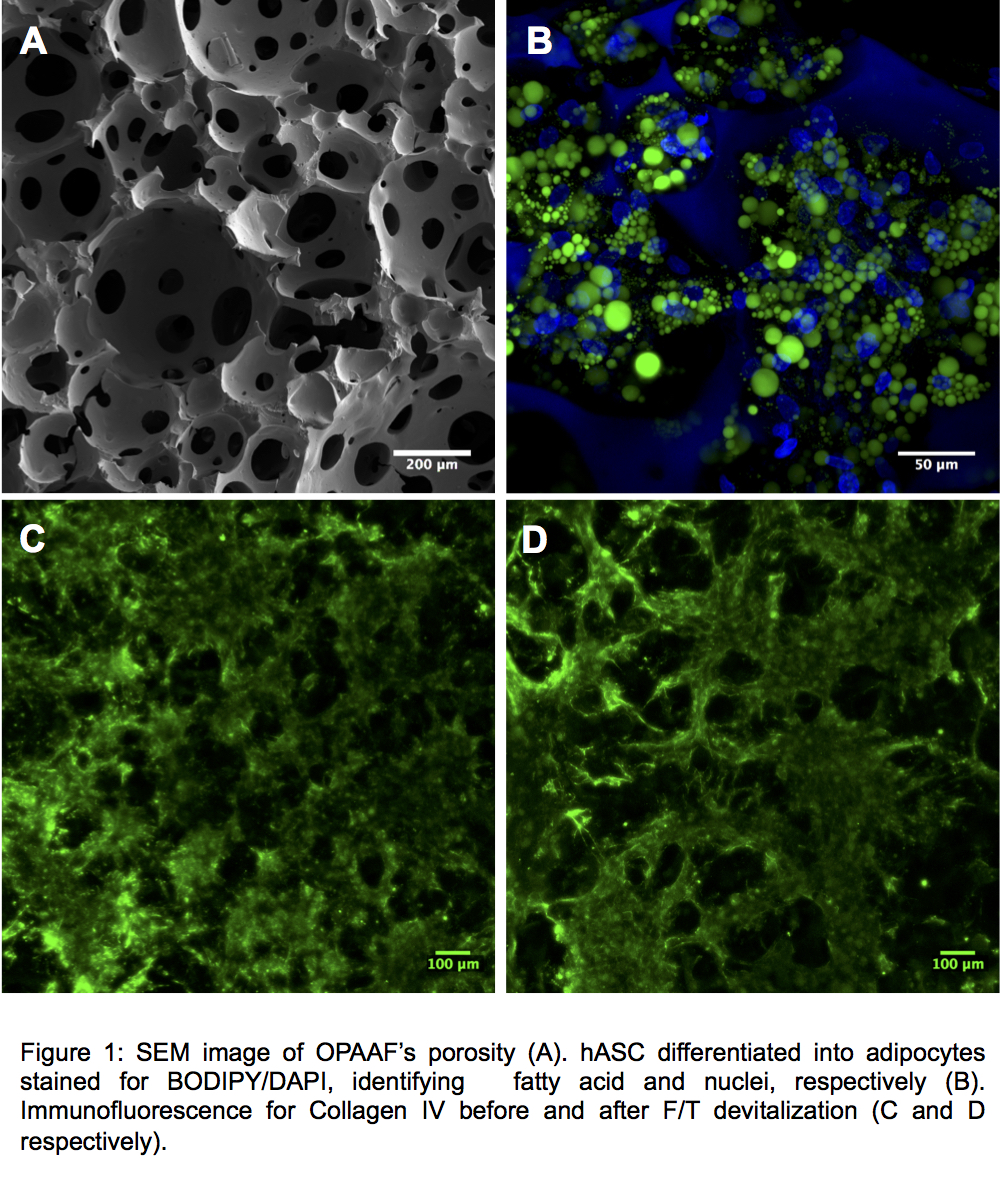
Results and Discussion: OPAAF was characterized by a highly interconnected porous network (Figure 1A) and mechanical properties (Equilibrium modulus 3.1 ± 0.5kPa) similar to those of a native adipose tissue. After 6 weeks in vivo, OPAAF was vascularized and colonized by adipose tissue. In in vitro perfusion culture experiments, OPAAF supported adipogenesis by hASCs, as documented by expression of the adipogenesis genes PPARg and FABP4 and by the formation of lipid droplets by hASC (Figure 1B). After devitalization of the constructs by F/T, followed by washings in the perfusion bioreactor system, a collagen IV analysis before and after devitalization (Figure C and D respectively) demonstrated the maintenance of the deposited ECM.
Conclusion: These results validated OPAAF as a scaffold suitable for adipose tissue engineering and repair. Moreover, OPAAF decorated with fat cell-deposited ECM were successfully generated, which could serve as off-the-shelf materials for soft tissue regeneration in defined volumes and shapes. Additional experiments, comparing the nude and ECM-decorated OPAAF, are ongoing to further characterize the deposited ECM and to investigate whether it can induce adipose tissue formation by mesenchymal progenitors both in vitro and in vivo.
Keywords:
Tissue Engineering,
3D scaffold,
Polymeric material,
acellullar matrix
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Biomaterials in constructing tissue substitutes
Citation:
Rossi
E,
Tocchio
A,
Gerges
I,
Scherberich
A,
Lenardi
C and
Martin
I
(2016). Decoration of RGD-mimetic porous scaffold with engineered, devitalized adipose matrix.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02432
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.