Bioengineered epicardial patch regenerates the adult mammalian heart
-
1
Stanford University School of Medicine, Stanford Cardiovascular Institute and Department of Pediatrics, United States
-
2
University of California, San Diego, Department of Bioengineering, United States
-
3
Sanford-Burnham-Prebys Medical Discovery Institute, United States
Adult mammals, unlike lower vertebrates, cannot effectively regenerate heart tissue, and myocyte loss following myocardial infarction (MI) is an important cause of human heart failure. Therefore, the elucidation of endogenous factors that control regeneration is of major scientific and therapeutic importance. Recent evidence suggests that the epicardium promotes myocardial regeneration; however, the mechanism remains largely unknown. In this study, we discovered that conditioned media from epicardial-like cultures enhance cardiomyogenesis in vitro[1]. We subsequently demonstrated that epicardial secreted factors embedded in a biomimetic type I collagen scaffold activate cardiomyocyte proliferation after ischemic heart injury (MI) in the mouse model (Figure1)[1]. An acellular type I collagen scaffold, endowed with specific physiomechanical properties approaching those of the embryonic epicardium, was generated utilizing plastic compression technique and used as cardiac patch (Figure 1a,b)[1]-[3].
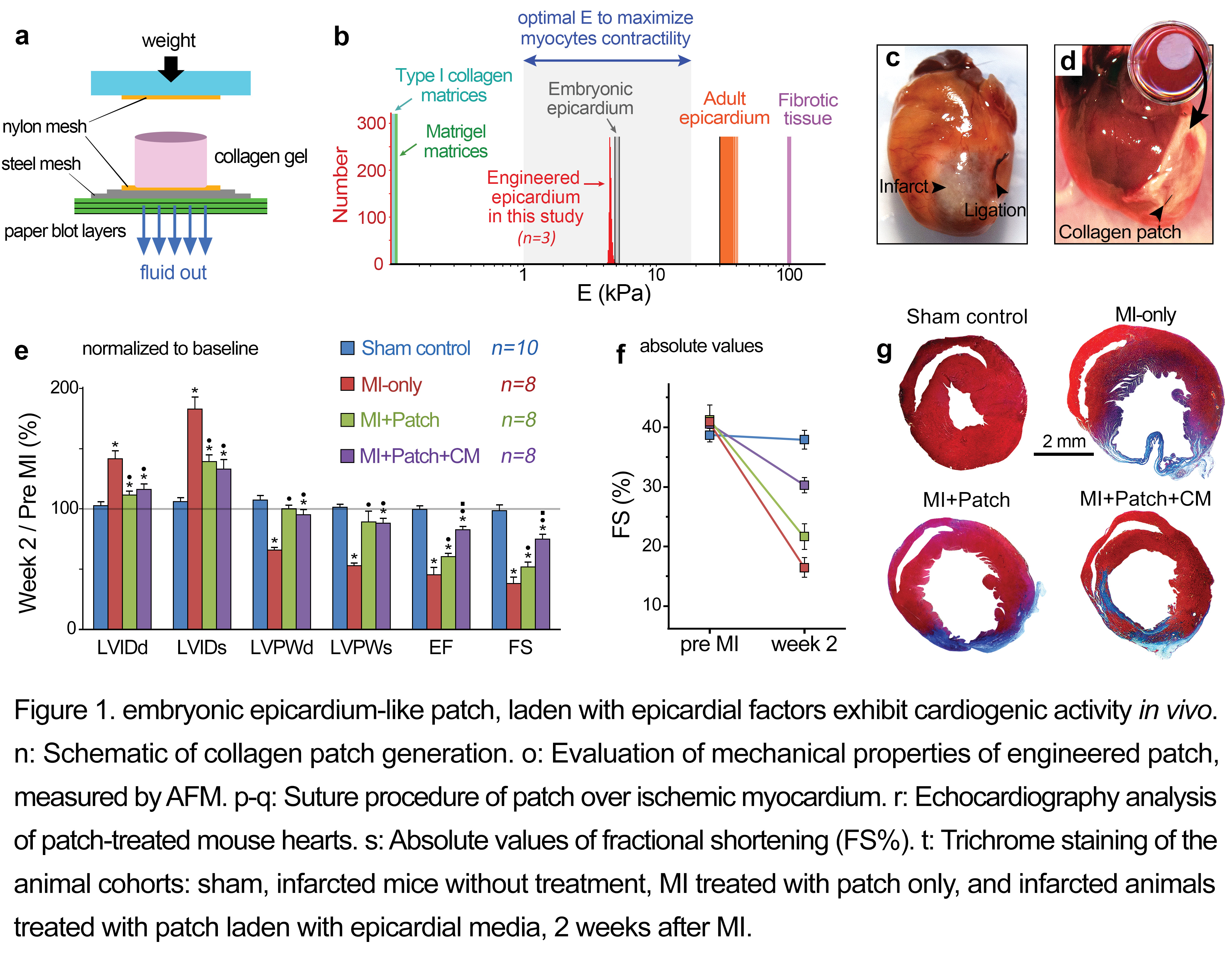
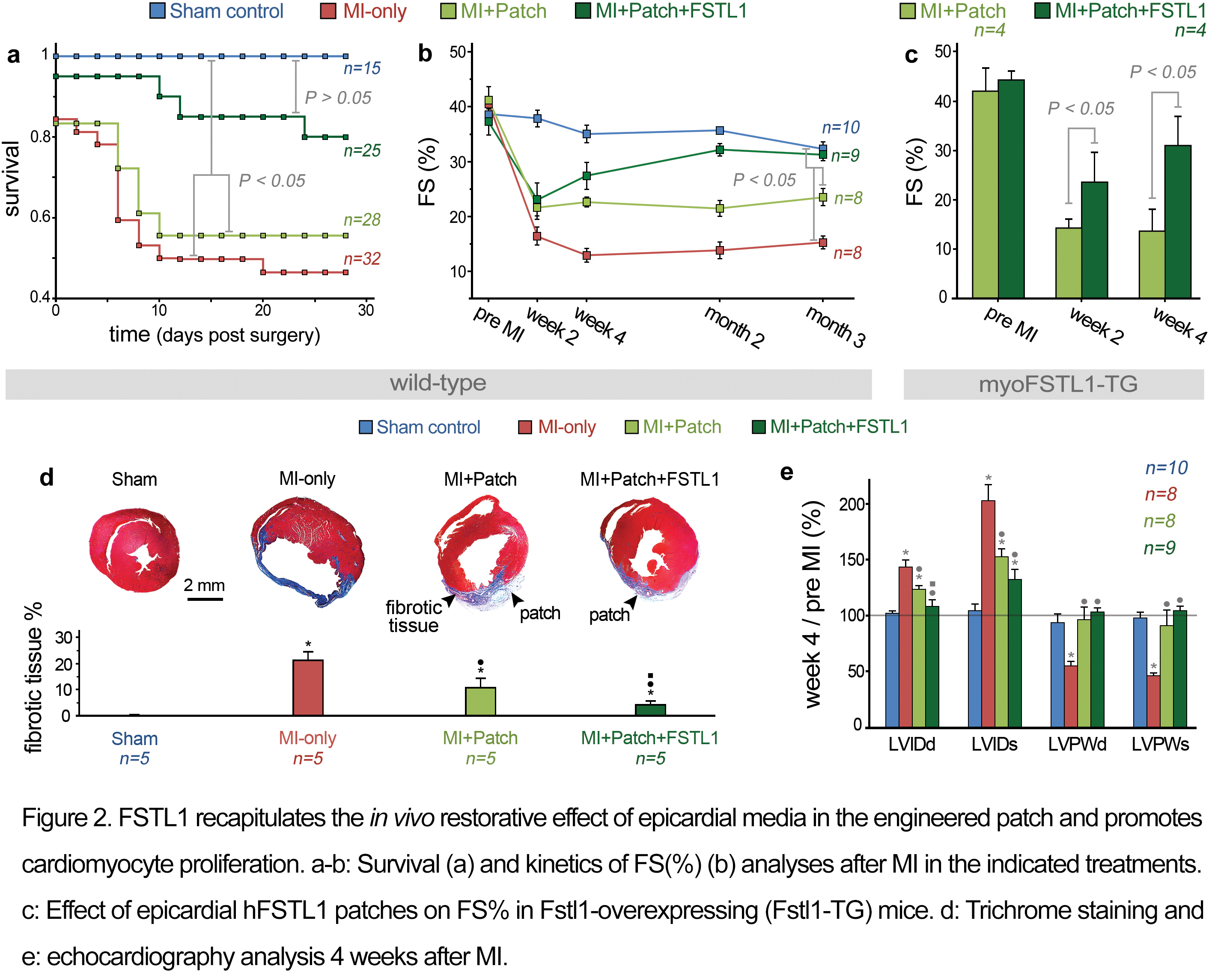
Mass Spectrometry analysis identified Follistatin-like 1 (FSTL1) protein as a main component of the epicardial-derived cardiogenic activity. Fstl1 is expressed in the adult epicardium but declines strikingly following MI[1]. Application of FSTL1 to the epicardial surface of the heart by a compressed collagen patch recapitulated the activity of epicardial-conditioned media and restored cardiac function in the mouse model (Figure 2). The engineered patch-FSTL1 treatment diminished pathological remodeling (ventricular dilation and fibrosis), restored vascularization, and induced cell cycle entry of pre-existing αMHC+ cells after MI, consequently improving long-term cardiac function (Figure 2)[1].
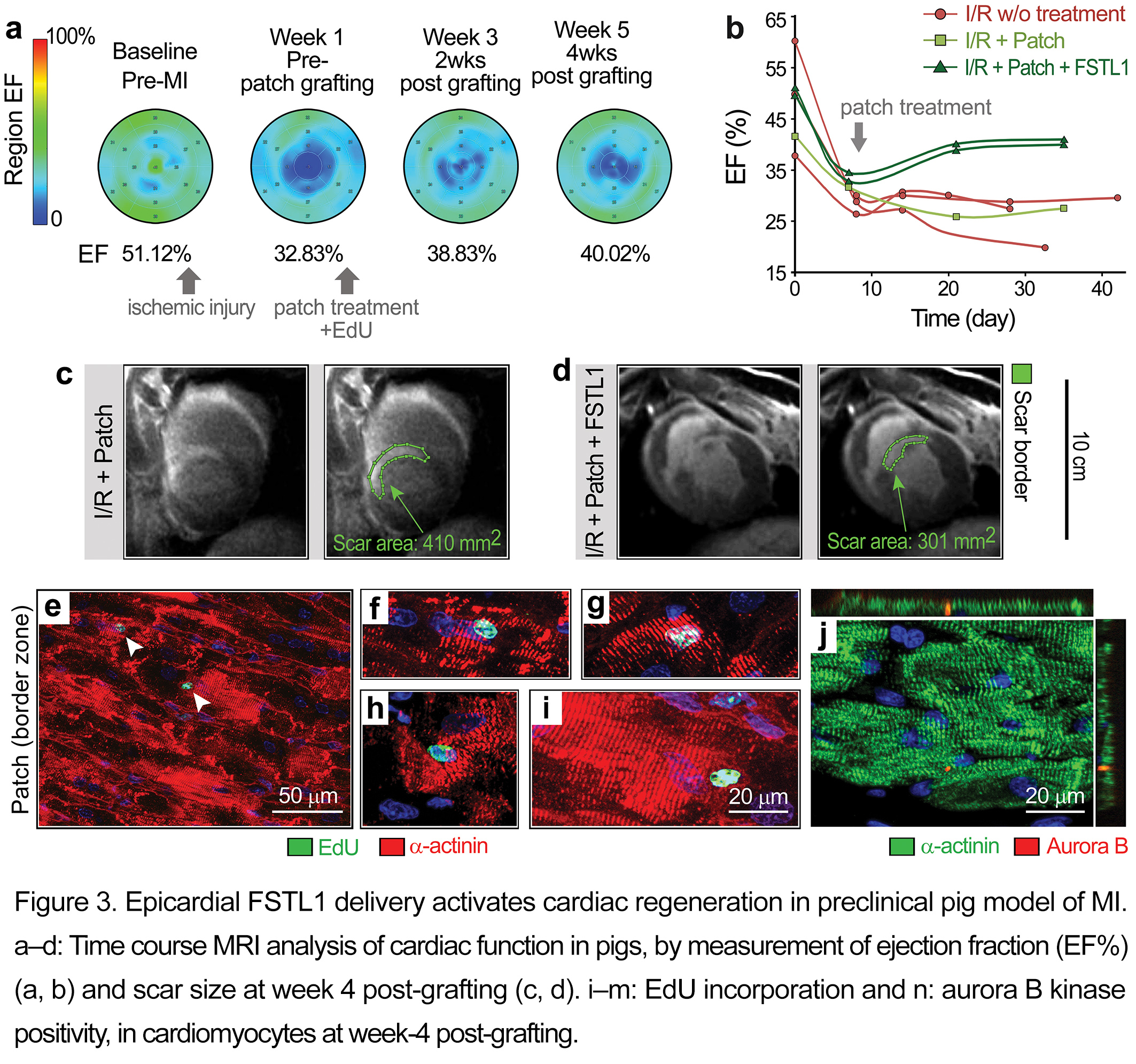
The epicardial patch-FSTL1 function was also evaluated in the swine model of ischemia-reperfusion (I/R) injury[1]. As determined by delayed enhancement MRI, application of the patch to the epicardium over the injured tissue at week 1 post-I/R stimulated recovery of contractile function (%EF) in 2 weeks. The recovery remained stable for an additional two weeks and was in contrast to the steady decline seen without treatment or following treatment with patch alone (Figure 3b). FSTL1-treated pigs demonstrated the least scar size of all treatments (green lines in Figure 3c-d). Immunostaining 4 weeks after patch implantation demonstrated cardiomyocytes in the border zone and ischemic area with evident EdU labelling and midbody-localized aurora B kinase (indicative of cytokinesis) (Figure 3e-j)[1].
References:
[1] K. Wei, V. Serpooshan (Co-First Author), et al. Nature 2015.
[2] V. Serpooshan and P. Ruiz-Lozano. Methods in Molecular Biology 2014.
[3] V. Serpooshan, et al., Biomaterials 2013.
Keywords:
Cell Proliferation,
in vivo tissue engineering,
Heart repair,
acellullar matrix
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Regenerative medicine: biomaterials for control of tissue induction
Citation:
Serpooshan
V,
Wei
K,
Mercola
M and
Ruiz-Lozano
P
(2016). Bioengineered epicardial patch regenerates the adult mammalian heart.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02401
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.