Introduction: The outward flow of blood during hemorrhage bleeding makes delivering coagulants to the site of bleeding a major challenge, and uncontrolled bleeding is a major cause of surgical complication and death[1],[2]. Self-propelling particles have been proposed as a strategy for transporting cargos against blood flow and have become a promising tool for designing agents which manage hemorrhage[3]. Self-propelling particles which can be loaded with procoagulant cargo can be used to transport these cargoes into sites of bleeding to rapidly halt hemorrage.
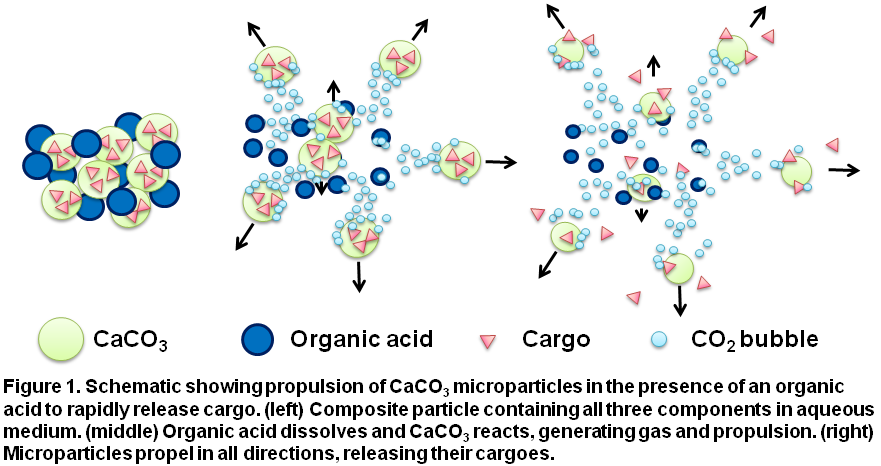
Materials and Methods: Self-propelling CaCO3 microparticles were synthesized via precipitation and solid-solid mixing with TXA. The velocity of particles propelling through blood and aqueous solutions was measured by optical microscopy. Thrombin was adsorbed onto microparticles by non-specific, aqueous-phase adsorption. Thrombin-loaded, self-propelling CaCO3 microparticles were tested for their ability to clot blood in vitro, in a mouse tail clip model of amputation and in a mouse liver puncture model of surgical bleeding. Self-propelling microparticles with TXA, but without thrombin, were also tested for their ability to reduce clot lysis and bleeding.
Results and Discussion: Self-propelling particles consisting of CaCO3 transported against flow through aqueous solutions and whole blood, up to millimetres into vasculature, at velocities of up to 1.5 cm s-1. Self-propelling CaCO3 microparticles, when loaded with thrombin, were an effective hemostatic agent and halted severe hemorrhage in both mouse models of bleeding.
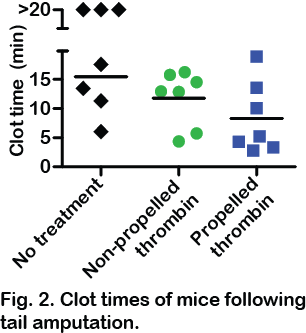
These particles were well tolerated and did not elicit excessive inflammation or local or systemic toxicity. Self-propelling CaCO3 microparticles loaded with TXA, but without thrombin, were also effective at reducing clot lysis and bleeding. These self-propelling, hemostatic particles may constitute an effective treatment for multiple bleeding scenarios including battlefield trauma and postpartum hemorrhage. Many medical applications have been proposed for self-propelling particles, and these findings show that self-propelled particles can function in vivo to enhance drug delivery.
Conclusion: We have successfully loaded self-propelling particles with TXA with or without thrombin to create two effective hemostatic agents. These particles burrowed into wound sites and reduced bleeding in vivo. This material has promising applications in managing multiple forms of hemorrhage, or for delivering other classes of therapeutics such as fibrinolytics.
Charles Haynes; Laura Ho; Esperanza Garcia; Stefanie Novakowski; UBC Animal Care Services; UBC Bioimaging Facility
References:
[1] Sauaia et al., J Trauma 38, 185-193 (1995).
[2] Kragh et al., US Army Med Dep J, 38-48 (2011).
[3] Zhao et al., Lab Chip 13, 1930-1936 (2013).