Introduction: About 25% of trauma patients experience impaired clotting ability due to a depletion of clotting factors during initial bleeding[1]. To combat these effects, human-derived products (whole blood, fresh frozen plasma, and factor concentrates) as well as recombinant factors are used as systemic treatments. While they improve patient outcomes, a number of limitations plague these treatments such as short shelf-lives, stringent storage conditions, microbial contamination risks, and the risk of immunogenicity in patients. Synthetic hemostats can improve clotting function while avoiding the limitations of current treatments. We recently reported a polymer-peptide conjugate known as PolySTAT.[2] PolySTAT mimics the activity of FXIIIa by crosslinking fibrin fibers through its multivalent display of fibrin-binding peptides (FBPs) (Fig. 1)[2]. In this work, we report structure-function studies leading to optimization of PolySTAT composition. We describe the performance of PolySTAT polymers in coagulation assays, characterization of PolySTAT-fibrin clots, and in vivo injury models in rats.
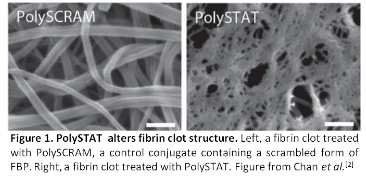
Materials and Methods: PolySTAT polymers were synthesized as described previously with varying peptide loading[2]. Polymers were characterized by GPC, NMR, and UV absorbance. Clotting parameters were measured in purified fibrin systems by rotational thromboelastometry (ROTEM). PolySTAT-treated fibrin clots were characterized by small-angle x-ray scattering (SAXS) and small-angle neutron scattering (SANS). PolySTAT polymers were evaluated in a rat model of trauma as described previously[2].
Results and Discussion: PolySTAT polymers with backbone repeat units of ~200 were synthesized with various peptide densities ranging from 0-10 peptides polymer polymer. In fibrin systems, a minimum peptide valency was determined for effective clot strengthening. Above this value of peptides per polymer, clotting parameters were similar. Specifically, PolySTAT treatment resulted in accelerated clotting kinetics, increased clot strength, and decreased clot lysis by ROTEM. SAXS confirmed that PolySTAT polymers did not aggregate in solution. Additionally, SANS showed that PolySTAT polymers did not significantly affect the self-assembly of fibrin at the fiber scale. Select polymers were tested in rat trauma models. PolySTAT treatment in rats reduced hemorrhage volumes and significantly improved survival.
Conclusions: We demonstrate that a minimum FBP valency for clot-enhancing effects was discovered in vitro. Select PolySTAT constructs were tested in vivo, and shown to reduce bleeding and improve survival in a rat femoral artery injury model.
This work was supported by NIH 1R21EB018637, NIH 2T32EB001650 (LWC & RJL) and an NSF GRFP (RJL).
References:
[1] Kauvar, D., et al. J. Trauma. (2006). 60: S3–S11
[2] Chan, L., et al. Sci. Transl. Med. (2015). 7: 277ra29