Introduction: Skeletal muscle has a robust capacity for repair in response to mild trauma[1]. However, when significant muscle volume is lost, regeneration is poor. Termed volumetric muscle loss (VML), the bulk loss (>20%) of muscle tissue overwhelms the capacity for repair, leading to the formation of scar tissue[2]. A promising repair strategy is restoration of the lost chemical and physical cues using decellularized skeletal muscle (DSM) scaffolds. However, a growing body of evidence suggests that DSM scaffolds alone are insufficient to stimulate muscle regeneration[3]. This study reports on the co-delivery of DSM scaffolds supplemented with minced muscle autografts as a treatment strategy for VML injury.
Materials and Methods:
With the aid of a biopsy punch, a partial thickness defect (8mm diameter x 3mm deep) representing approximately 20% of the tibialis anterior (TA) muscle mass was created in Fisher 344 rats. Animals, were assigned to one of three groups; unrepaired defect, defect repaired with DSM alone, or defect repair with combined DSM+MM implants (n=8/group). The contralateral limb remained was untreated to serve as a normal control. DSM implants were prepared from TA muscles collected from rats which had been euthanized as part of an unrelated study. Minced muscle was prepared using 25% of the muscle harvested from the defect site.
At the completion of a 12-week recovery period, TA muscle peak tetanic contractile force was measured in situ for all treatment groups. Tissues were cryo-sectioned and immunostained with antibodies directed against collagen I, collagen III, and myosin heavy chain. The expression of MyoD, Collagen I, Collagen III, CTGF, TGF-β, and Timp-1 genes within cells collected from the defect/repair site was measured using RT-PCR.
Results and Discussion: VML repair with combined DSM+MM implants significantly improved peak contractile force (1.66±0.08 N/kg) when compared to unrepaired controls (1.39±0.11 N/kg) or muscles repaired with DSM alone (1.54±0.10 N/kg).
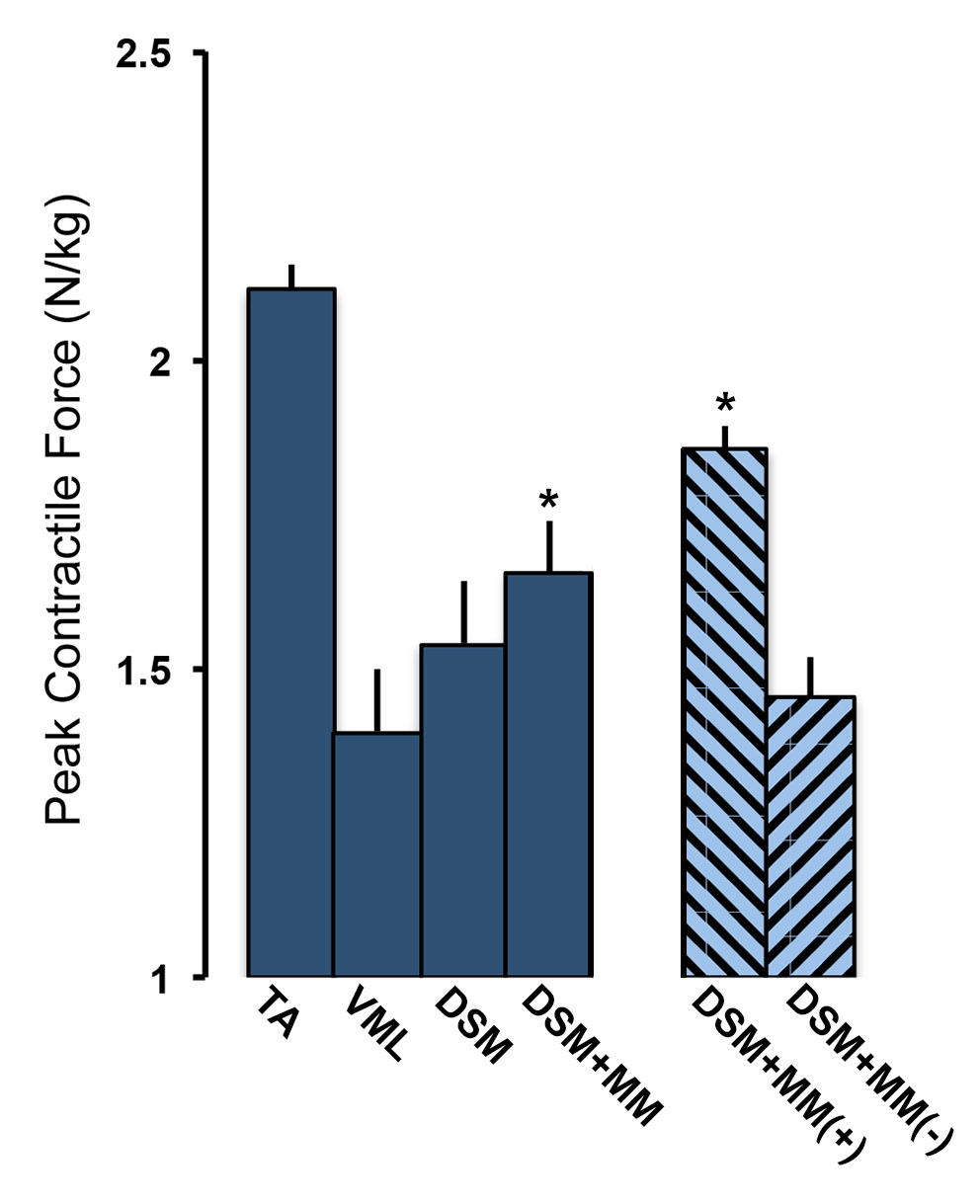
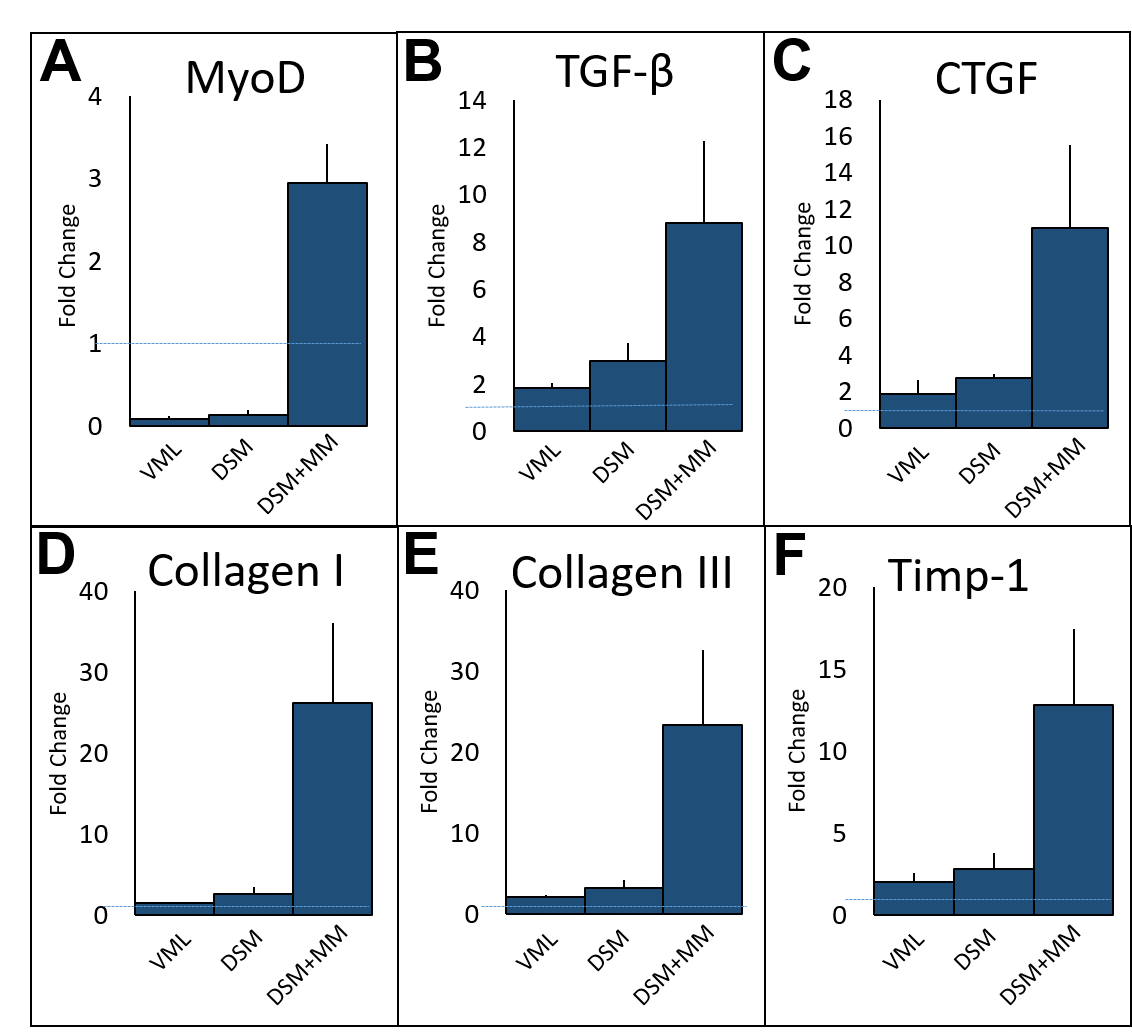
The contractile force results were consistent with histological findings showing a marked decrease in fibrosis at the defect sites of DSM+MM repaired muscles. Myogenic and extracellular matrix gene production was dramatically increased in tissue repaired with DSM+MM implants. Most notably, treatment with DSM+MM resulted in a three-fold increase in the expression of the myogenesis marker, MyoD, when compared to normal muscle, suggesting active muscle myogenesis. By comparison, MyoD expression was decreased in both no repair and isolated DSM repair treatment groups.
Conclusion: The findings of this study suggest that supplementation of DSM implants with MM is a viable strategy for the repair of VML injuries. While the clinical use of an all MM implant would be limited by donor site morbidity, the supplementation of DSM implants with a limited volume of minced muscle slurry harvested via biopsy from healthy tissue could be a viable approach.
National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH Award# R15AR064481); Arkansas Bioscience Institute
References:
[1] Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res 2012;347:759-774.
[2] Grogan BF, Hsu JR, Skeletal Trauma Research Consortium. Volumetric muscle loss. J Am Acad Orthop Surg 2011;19 Suppl 1:S35-7.
[3] Corona BT, Wu X, Ward CL, McDaniel JS, Rathbone CR, Walters TJ. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials 2013;34:3324-3335.