Introduction: Iron is an important element that plays a vital role in the metabolism of human body[1]-[3]. Iron overload due to genetic diseases, hereditary haemochromatosis, or transfusion hemosiderosis is a disorder that causes the body to absorb more iron than normal[4],[5]. The extra absorbed iron accumulates in vital organs such as liver, heart, pancreas over time, leading them to fail if not treated[6]. Current gold standard in the treatment iron overload includes using therapeutic agents such as deferoxamine (DFO) and diet modification. DFO in patients with iron overload disorder is commonly administered by subcutaneous infusion using a syringe pump over the course of 8–12 hrs[4],[7], which causes discomfort such as swelling, itching, and pain in the infusion sit for the patient. Niosomes are vesicular systems that are composed of non-ionic surfactants and cholesterol and are biodegradable, biocompatible, and can be produced in a low cost process[8],[9]. Here, we use niosomes as carriers for delivery of DFO and show that using this approach improves the sustained delivery of this drug and can be potentially used for treating patients with Iron overload disorder (Fig1.a).
Materials and Methods: Drug release studies were performed by dialysis bag method. The size of the nanoparticles was determined by transmission electron microscopy (TEM). For evaluating Iron chelation efficacy of nano-niosomal DFO in comparison with free DFO, iron induced to Hep-G2 cell lines and after cell iron overloading, were treated by free drug and formulated drug and intracellular iron chelation efficacy measured by atomic absorption spectrometry.
Results and Discussion: TEM images reveal that the nano-Niosomal DFO particles are almost spherical and have a diameter of 150 nm (Fig. 1b). The release study shows a more sustained release of the drug from niosomes compared to the free drug (Fig. 1c). Moreover, iron chelation efficacy in nano-niosomal DFO was significantly improved in chelation of intracellular iron as compared to the free drug (Fig. 1d). Our results suggest that niosomes loaded with DFO is an alternative to the current subcutaneous injection of the drug, which can significantly improve the treatment of patients with iron overload disorders.
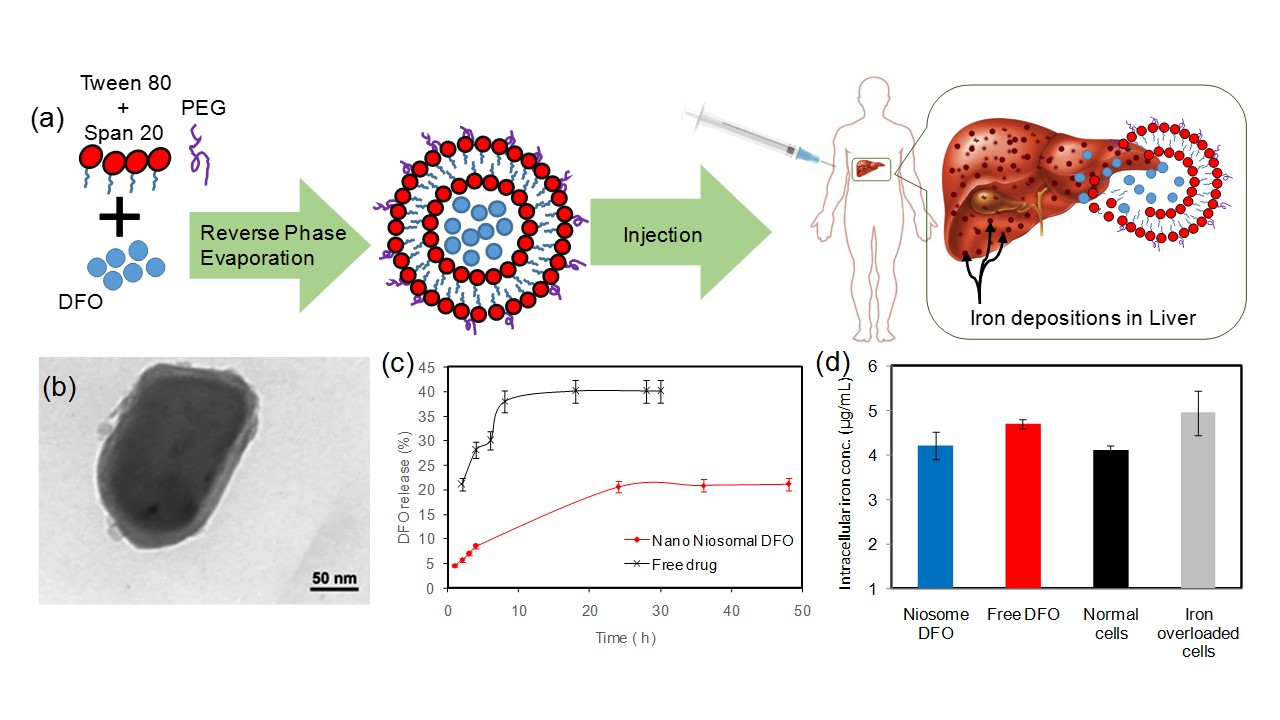
Figure 1. Niosomes loaded with DFO for treatment of iron overload disorders. (a) Schematic of the process; (b) TEM image of a niosome particle; (c) release profile of the free drug and niosomal DFO; (d) Intra cellular iron chelation efficiency on liver cells.
Conclusion: Commercial Deferoxamine (DFO) is one of the most common drugs that used for iron chelating in patients with iron overload. Commercial DFO has several drawbacks including some serious problem and needs long time infusion. In this paper, a novel nanocarrier system was introduced to improve stability and controlled release of drug, DFO, for intravenous administration. We showed sustained release of DFO from niosomes and higher chelation efficiency compared to the free drug. It is concluded that prepared Niosome nanoparticles introduced here could be a promising clinical intravenous system for delivery Iron chelating drug such as DFO.
References:
[1] Srai S, Bomford A, McArdle HJ. Iron transport across cell membranes: molecular understanding of duodenal and placental iron uptake. Best Practice & Research Clinical Haematology. 2002;15(2):243-59.
[2] Sripetchwandee J, Pipatpiboon N, Chattipakorn N, Chattipakorn S. Combined Therapy of Iron Chelator and Antioxidant Completely Restores Brain Dysfunction Induced by Iron Toxicity. PLoS ONE. 2014;9(1):e85115.
[3] Weiss G, Werner-Felmayer G, Werner ER, Grunewald K, Wachter H, Hentze MW. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. The Journal of experimental medicine. 1994;180(3):969-76.
[4] Kontoghiorghes GJ, Pattichi K, Hadjigavriel M, Kolnagou A. Transfusional iron overload and chelation therapy with deferoxamine and deferiprone (L1). Transfusion science. 2000;23(3):211-23.
[5] Cappellini MD. Exjade® (deferasirox, ICL670) in the treatment of chronic iron overload associated with blood transfusion. Therapeutics and Clinical Risk Management. 2007;3(2):291-9.
[6] Jain N. Essentials of hematology. Lea & Febiger, Philadelphia, Pa, USA. 1993.
[7] Vermylen C. What is new in iron overload? European Journal of Pediatrics. 2008;167(4):377-81.
[8] Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. Journal of Controlled Release. 2014;185(0):22-36.
[9] Kumar GP, Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharmaceutica Sinica B. 2011;1(4):208-19.