Hemorrhage control is a major focus in treating critically injured trauma patients. Uncontrolled blood loss accounts for 25-30% of civilian deaths and the vast majority of all battlefield deaths. Acute traumatic coagulopathy (ATC) occurs in 25-40% of patients nearly immediately after injury and is associated with worsened bleeding, increased need for transfusion, and a 4-fold increase in mortality. Stopping bleeding and correcting coagulopathy remain the key goals in trauma treatment. Rapid effectiveness of both is the key to saving lives and preventing morbidity.
Unfortunately, current treatments are few and woefully inadequate due to ineffectiveness, side effects, steep cost per dose, and need for refrigeration. Even when effective, there is an inherent delay between injury and the use of recombinant protein hemostats due to transporting a patient to the hospital. Biomaterials can bridge the critical time between injury and treatment to begin treatment quicker and improve the condition of trauma patients upon hospital arrival. One such potential solution is created by coating a silica nanoparticle (SNP) with a heterogeneous 70-mer polymer polyphosphate (polyP) chain to form polyP-SNP.
In response to vessel injury, short-chain polyP binds to thrombin at the injury site. After binding to thrombin, polyP enhances back-activation of FXI and FV, ultimately leading to an increase in the production of thrombin, the integral factor for clotting. Increased production of thrombin quickly leads to the formation of the physical clot which seals the wound and limits blood loss. However, short-chain polyP is a poor activator of FXII, which makes it a potentially ideal agent for enhancing the body’s ability to clot at injury sites without inducing clots in otherwise healthy vessels. Among its many benefits, the long term stability of polyP-SNP at ambient conditions has the potential to minimize this time between injury and treatment.
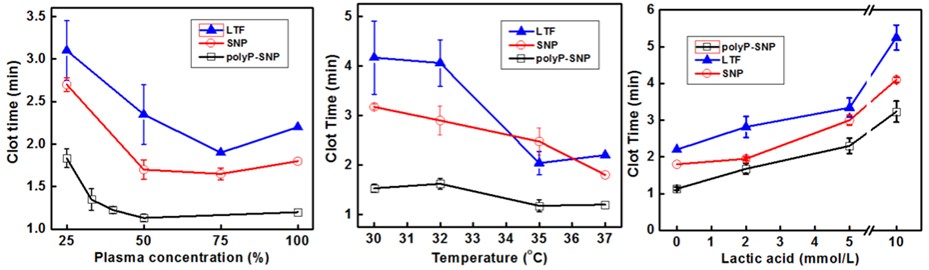
Along with ATC, an iatrogenic coagulopathy, which encompasses hemodilution, factor depletion, hypothermia, and metabolic acidosis, often occurs as a result of resuscitative efforts. We have conducted in vitro studies to determine the efficacy of our polyP-SNP particles under hemodilute, hypothermic, and acidotic conditions. The use of polyP-SNP improved coagulation dynamics in all three conditions (Fig. 1). This in vitro data using simulated trauma conditions suggests its potential efficacy in future in vivo trials.
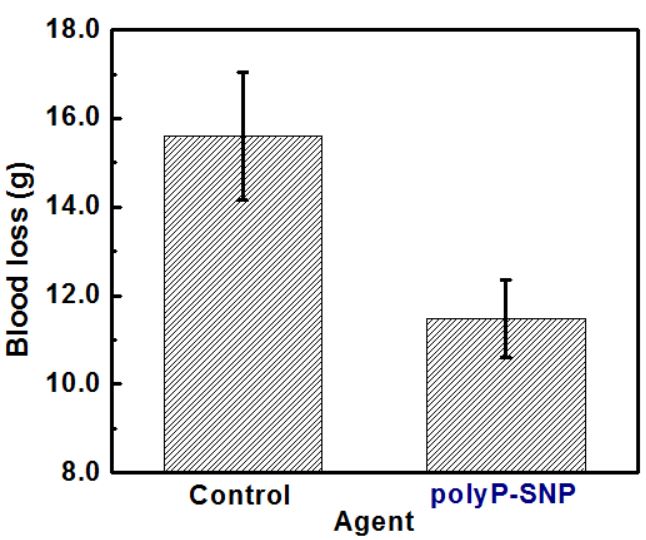
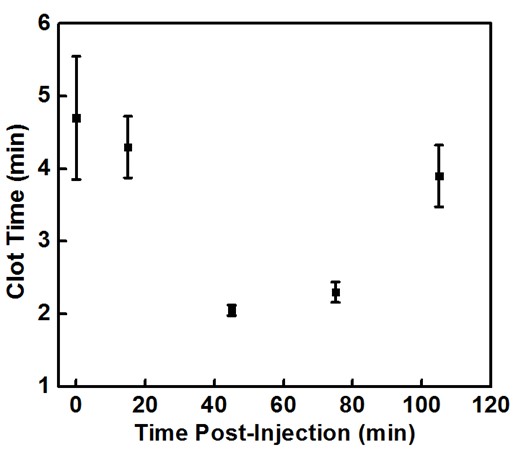
Preliminary animal trials suggests that a 5 mg/kg dose of polyP-SNPs after a tail cut reduced blood loss by roughly 25 % (Fig. 2, p = 0.011) and led to 100 % survival. Earlier tests conducted to determine the circulation time of polyP-SNPs using blood drawn from an uninjured rat after a lower dose (2 mg/kg) injection. In the presence of injury, these tests showed that the polyP-SNP particles are active in the bloodstream for roughly 2 hours after injection (Fig. 3). Post-euthanasia histopathology studies showed that polyP-SNPs were not thrombotically active in uninjured vessels during the same time period. This suggests that the polyP-SNP system is both safe and effective for intravenous use in an animal model of trauma.
PolyP-SNP’s ability to quickly grow clots is extremely valuable given Hagemo et al.’s findings that fibrinogen levels and fibrin clot size after 5 minutes were the best predictors of coagulopathy and need for massive transfusion in trauma patients. The stability of polyP-SNPs at ambient conditions also enables for quicker intervention to prevent or attenuate shock and the onset of ATC. Initial in vivo studies have shown that the use of polyP-SNP quickly after injury significantly reduces blood loss when medical personnel have few viable options. This system has been shown to be effective at reducing blood loss in a traumatic injury without unwanted clotting, while also reducing clot time in simulated trauma. PolyP-SNP have the potential to be at the vanguard of biomaterials that save lives by safely and effectively enabling prehospital treatment for trauma patients.