Anti-infective coatings for titanium implants by alternating current electrophoretic deposition
-
1
KU Leuven, Department of Materials Engineering, Belgium
-
2
KU Leuven, Centre of Microbial and Plant Genetics, Belgium
-
3
Friedrich-Alexander-University, Department of Materials Science and Engineering, Germany
Introduction: Artificial implant surfaces are ideal substrates for microbial attachment and subsequent biofilm formation. Within this protective biofilm environment, microbes are highly tolerant to conventional antimicrobial therapy leading to recalcitrant infections[1]. New strategies to protect the implant surface against biofilm formation are needed. One strategy is the immobilization of antimicrobials on the implant surface as such non-releasing coatings can prevent microbial colonization of the coated implant material over a prolonged period of time.
However, customary chemical grafting by immersion of the implant material in a concentrated biomolecule solution is a time-consuming process (hours to days)[2]. In this study, we propose a faster alternative for the deposition of biomolecules using alternating current electrophoretic deposition (AC-EPD). By applying an asymmetrically alternating electric field, biomolecules can be deposited at the electrode surface within minutes without compromising their bioactivity. Here, we applied AC-EPD for the deposition of the antimicrobial agent caspofungin on titanium and tested the in vitro potential of this coated material to resist colonization by the fungal pathogen Candida albicans (C. albicans).
Materials and Methods: Titanium electrodes (cp Ti, grade 2) were silanized (3-aminopropyltriethoxysilane), activated with isocyanate groups (hexamethylene diisocyanate) and submerged vertically in an aqueous 900 µM caspofungin (Cancidas) solution. AC-EPD was performed in a controlled-current mode, applying a triangular AC-signal having an asymmetry of 4[3], with a 10 mA amplitude and a 50 Hz frequency superimposed with a mild 0.1 mA DC offset for 10 min. After rinsing and drying, coatings were evaluated by scanning electron microscopy (SEM), time-of-flight secondary ion mass spectroscopy (ToF-SIMS) and micro bicinchoninic acid (BCA) protein assay. Furthermore, discs were incubated for 24 h at 37°C with RPMI containing 5 x 104 cells/ml C. albicans. Biofilms were quantified by colony forming unit (cfu) counting and visualized using SEM.
Results and Discussion: SEM observation showed a selective deposition on the electrode coupled as cathode during the high amplitude peak, while the anode showed the pristine Ti surface (figure 1). ToF-SIMS analysis confirmed the presence of caspofungin at the cathode, while strong Ti substrate signals were collected for the counterelectrode. This is in agreement with the positive charge of caspofungin at natural pH. Analysis of protein content via the BCA assay indicated a higher deposition yield in case of AC-EPD as compared to immersion or even direct current EPD in combination with surface activation. The amount of biofilm cells (assessed as cfu) and the structural integrity of the biofilm (visualized via SEM) present on the caspofungin-coated titanium substrates was significantly reduced pointing to preservation of caspofungin’s antifungal activity upon the AC-EPD process.
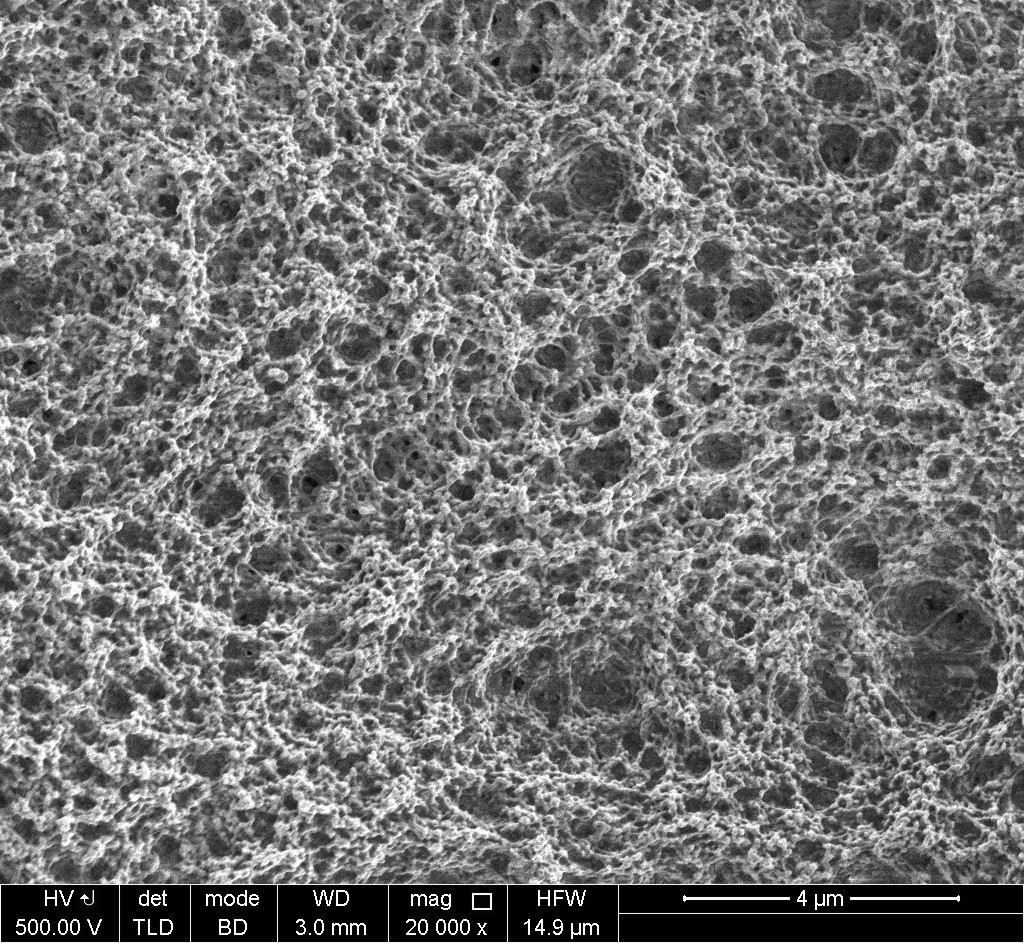
Figure 1: SEM micrograph of a Ti cathode after caspofungin deposition using AC-EPD.
Conclusion: A proof-of-principle was established for the deposition of caspofungin on titanium using AC-EPD in combination with surface activation that showed activity against C. albicans biofilm formation in vitro. The reduced processing time is an important progress in the immobilization of bioactive molecules.
The research leading to these results has received funding from the European Commission’s Seventh Framework Program (FP7/2007-2013) under the grant agreement COATIM (project n° 278425), the Industrial Research Fund of KU Leuven by the knowledge platform IOF/KP/11/007 and the Flemish government via the Hercules Foundation (project ZW09-09). A.B. acknowledges the receipt of a travel grant from FWO-Vlaanderen (K229615N); B.N. received a postdoctoral grant from FWO-Vlaanderen (1.2.B62.12N) and a YouReCa Junior Mobility grant (JUMO/14/024); K.T. is an industrial research manager supported by the Industrial Research Fund (IOFm/05/022, KU Leuven). The funders had no involvement in study design; collection, analysis and interpretation of data; writing of the report; neither in the decision to submit the article for publication.
References:
[1] Edmiston CE, McBain AJ, Roberts C and Leaper D. Clinical and microbiological aspects of biofilm-associated surgical site infections. Adv Exp Med Biol 2015; 830: 47–67.
[2] Godoy-Gallardo M, Mas-Moruno C, Fernández-Calderón MC, Pérez-Giraldo C, Manero JM, Albericio F, Gil FJ and Rodríguez D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater 2014; 10: 3522-3534.
[3] Neirinck B, Fransaer J, Van der Biest O, Vleugels J. Aqueous electrophoretic deposition in asymmetric AC electric fields (AC-EPD). Electrochem Commun 2009; 11: 57-60.
Keywords:
Infection,
electric,
Surface modification,
biofunctionalization
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Anti-infective biomaterials
Citation:
Braem
A,
Neirinck
B,
De Brucker
K,
Killian
MS,
Virtanen
S,
Thevissen
K,
Cammue
BP and
Vleugels
J
(2016). Anti-infective coatings for titanium implants by alternating current electrophoretic deposition.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02118
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Annabel Braem, KU Leuven, Department of Materials Engineering, Leuven, Belgium, Email1
Dr. Bram Neirinck, KU Leuven, Department of Materials Engineering, Leuven, Belgium, Email2
Dr. Manuela S Killian, Friedrich-Alexander-University, Department of Materials Science and Engineering, Erlangen-Nürnberg, Germany, Email3
Dr. Sannakaisa Virtanen, Friedrich-Alexander-University, Department of Materials Science and Engineering, Erlangen-Nürnberg, Germany, Email4
Dr. Karin Thevissen, KU Leuven, Centre of Microbial and Plant Genetics, Leuven, Belgium, Email5
Dr. Bruno P Cammue, KU Leuven, Centre of Microbial and Plant Genetics, Leuven, Belgium, Email6
Dr. Jef Vleugels, KU Leuven, Department of Materials Engineering, Leuven, Belgium, Email7