Biocompatible mechanical surface modification processing and mechanical properties of beta-type titanium alloys
-
1
Meijo University, Graduate School of Science and Technology, Japan
-
2
Meijo University, Faculty of Science and Technology, Japan
-
3
Tohoku University, Faculty of Science and Technology, Japan
-
4
Suzuka National College of Technology, Dept. of Mechanical Engineering, Japan
-
5
Kobe University, Dept. of Mechanical Engineering, Japan
Introduction: Newly developed β-type Ti-29Nb-13Ta-4.6Zr (TNTZ) is one of representative biomedical Ti alloys with high biocompatibility and a relatively low elastic modulus of around 60 GPa close to that of the cortical bone. However, TNTZ doesn’t show the direct contact with the bone while biomedical ceramics such as hydroxyapatite (HAp) easily contacts with that. Therefore, the formation of bioactive layer on the surface of TNTZ by HAp fine particle bomberding (HAp FPB) was investigated in this study. In addition, HAp FPB after glass-beads FPB was also done for effective improvement of mechanical properties of TNTZ.
Experimental Methods: Materials used in this study were TNTZ subjected to a solution treatment (ST) at 1063 K for 3.6 ks in a vacuum followed by water quenching (WQ), which are designated as TNTZST. Some samples were subjected to aging treatment at 673 K for 1.2 ks after ST, which are designated as TNTZAT673 K. Surface modification by HAp fine particle bomberding (HAp FPB) for 10 and 20 s was conducted with all samples as mentioned above, which are designated as TNTZST/HAp and TNTZAT/HAp, respectively. Annealed Ti-6Al-4V was used as a comparison material (Ti64annealed). Ti64annealed was also subjected to HAp FPB in the same condition for TNTZ, which is designated as Ti64/HAp. Some samples of TNTZST and Ti64annealed were done to HAp FPB after glass-beads FPB. Optical microscopy (OM), scanning electron microscopy (SEM), and X-ray diffraction (XRD) spectroscopy were carried out on each specimen to evaluate the microstructure. Vickers hardness (HV) and fatigue tests were performed to examine the mechanical properties.
Results and Discussion: HV of TNTZST/HAp for 10 and 20 s, TNTZAT/HAp for 10 and 20 s and Ti64/HAp for 10 and 20 s increased slightly near the specimen surface as compared with that of TNTZST, TNTZAT673 K and Ti64annealed. HV profile of TNTZST/HAp for 10 s and TNTZST/HAp for 20 s as representatively shown in Fig 1 . have a hardened region of HV from the specimen surface to around 100 μm in depth. The increased value of HV on TNTZST/HAp is around 30 HV higher as compared to that of TNTZST. According to an increase in treatment time for FPB, the thickness of harden layer increases slightly. Figure 2 shows S-N curves obtained from fatigue tests on TNTZST and TNTZST/HAp for 20 s. The fatigue strength of TNTZST/HAp for 20 s increases in the high cycle fatigue life region. The fatigue limit of TNTZST/HAp for 20 s is around 300 MPa and around 60 MPa higher than that of TNTZST. HV near specimen surface on TNTZST/HAp for 20 s was around 40 HV higher than that of center of specimen as mentioned above. As a result, it is considered that the effect of residual compressive stress by HAp FPB occurred in high cycle fatigue life region.
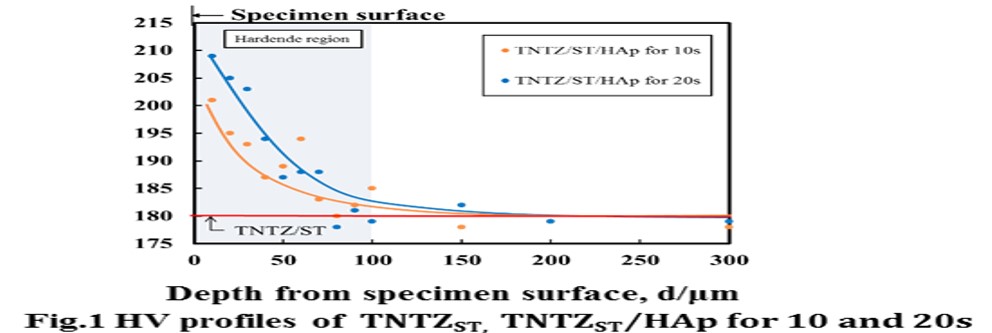
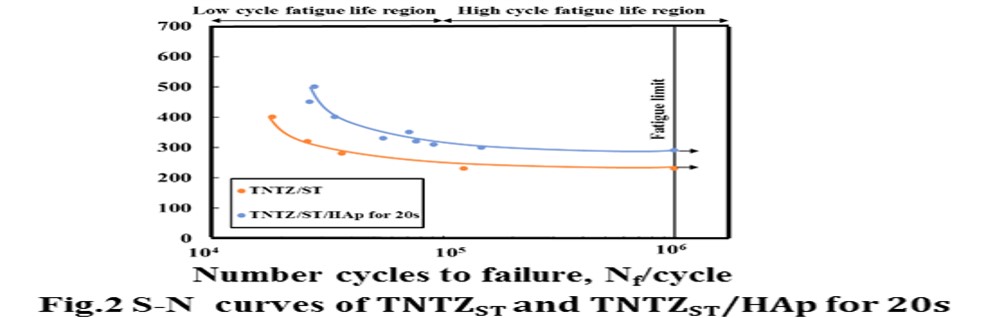
Conclusion: (1) HV of TNTZST subjected to HAp FPB showed the maximum value in very edge of specimen surface. In particular, HV near the specimen surface of TNTZST/HAp 20 s increased by around 30 HV as compared to that of TNTZST. (2) Fatigue strength of TNTZST subjected to HAp FPB increased in high cycle fatigue life region. The fatigue limit was around 300 MPa.
Keywords:
microstructure,
mechanical property
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Mechanical properties of biomaterials
Citation:
Ban
A,
Sato
M,
Akahori
T,
Hattori
T,
Niinomi
M,
Nambu
K and
Kikuchi
S
(2016). Biocompatible mechanical surface modification processing and mechanical properties of beta-type titanium alloys.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02091
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Koichiro Nambu, Suzuka National College of Technology, Dept. of Mechanical Engineering, Suzuka, Japan, Email1