Introduction: More than 50% of dental restorations placed annually are resin composites[1]. However, these materials experience significant biological breakdown in the oral cavity that is believed to be facilitated by oral bacteria which migrate within the margin between the tooth and restorative material. Ciprofloxacin (CF) and Metronidazole (MN) are two antimicrobial agents with potential to manage oral bacterial in the margin environment[2],[3] Previous work reported on the synthesis of CF derived vinyl monomers for adhesives [4]. The objectives of the current work are to synthesize an antimicrobial di-vinyl monomer that will release MN upon hydrolytic degradation, and to formulate adhesive systems containing a combination of both MN and CF derived di-vinyl monomers.
Methods: A lysine/hydroxyl-methyl methacrylate (HLH) derivative was synthesized [5]. Using carbodiimide chemistry, the hydroxyl moiety of MN was coupled to HLH acidified ester, yielding HLH-MN. The monomer was characterized by nuclear magnetic resonance. Biodegradation studies (0.1mM) of HLH, HLH-MN and commercial Bisphenol A-glycidyl methacrylate (BisGMA) (n=9, repeated 3 times) were carried out at 37⁰C in buffer or enzyme solution (containing cholesterol esterase activity and pseudocholinesterase activity) for 4 days and solutions characterized by high performance liquid chromatography (HPLC)[5],[6]. Preliminary formulations of CF and MN derived monomers were light cured (1min) using camphorquinone/2-(dimethyl-amino) ethyl methacrylate photo-initiator system, with commercial monomers BisGMA and 2-hydroxyethyl methacrylate (HEMA)[4]. Gel contents were determined and cell toxicity compared using human gingival fibroblasts.
Results and Discussion: HLH-MN structure was confirmed by 1H-NMR (Fig 1). The amount of MN released was not significantly different between the buffer or enzyme condition (Fig 2a), whereas it was for BisGMA[7]. HLH-MN was more stable than BisGMA under enzymatic conditions (Fig 2b). The rate of MN released in buffer (4.091 µM/day, r2 = 0.99) was much greater than CF (0.039 µM/day, r2 = 0.86). The linkage between the backbone and MN is an ester while CF is coupled via a more stable amide. This will play to the advantage of each of the drugs relative to minimum inhibitory concentrations (MICs) for Streptococcus mutans, (0.7 µg/mL for CF vs 700 µg/mL for MN). The gel content for cured adhesives with weight percent values for MN:CF:Hema:BisGMA of 5:10:30:55; 10:5:30:55, and 15:15:15:55 were 99.2% (±0.5), 99.0% (±0.7) and 96.0% (±2.8), respectively. These high conversion states are anticipated to yield good cell compatibility in on-going work.
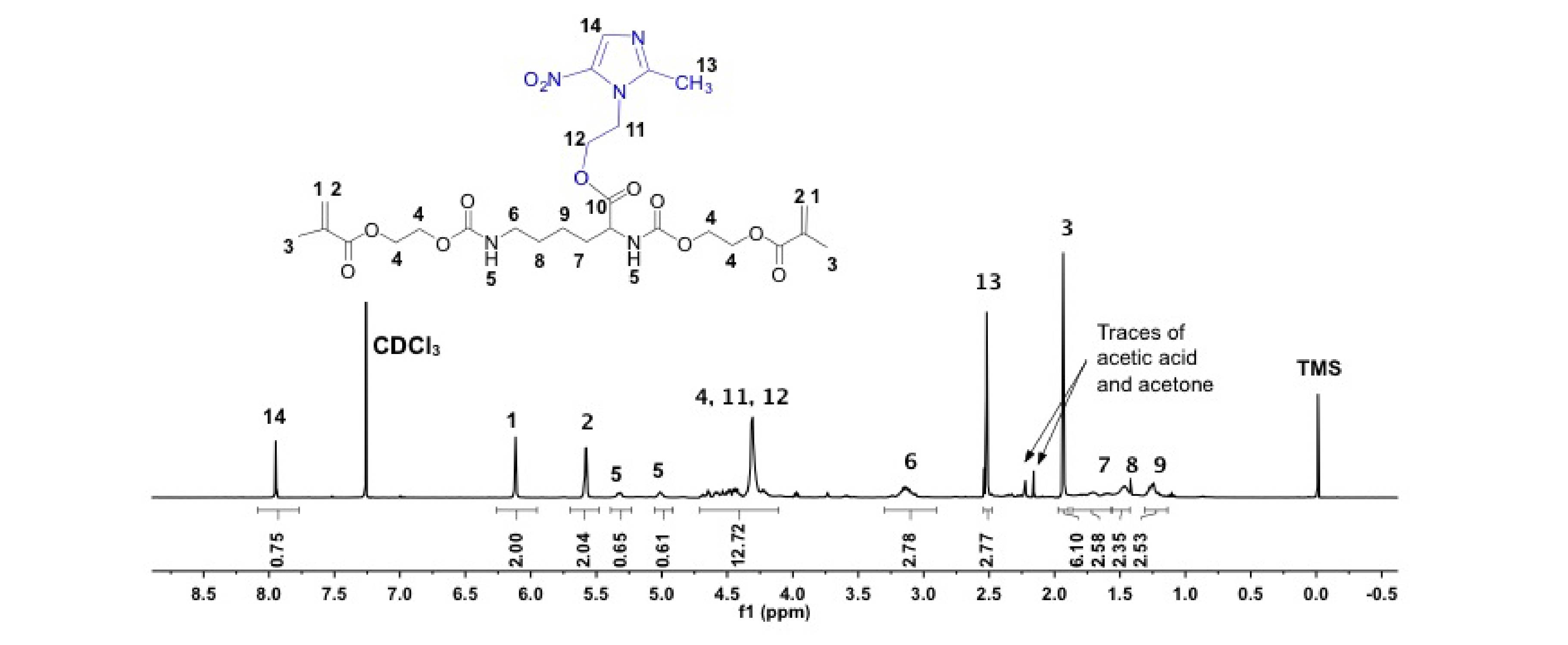
Figure 1. 1H-NMR(CDCl3, 400MHz) of HLH-MN, the drug MN is highlighted in blue.
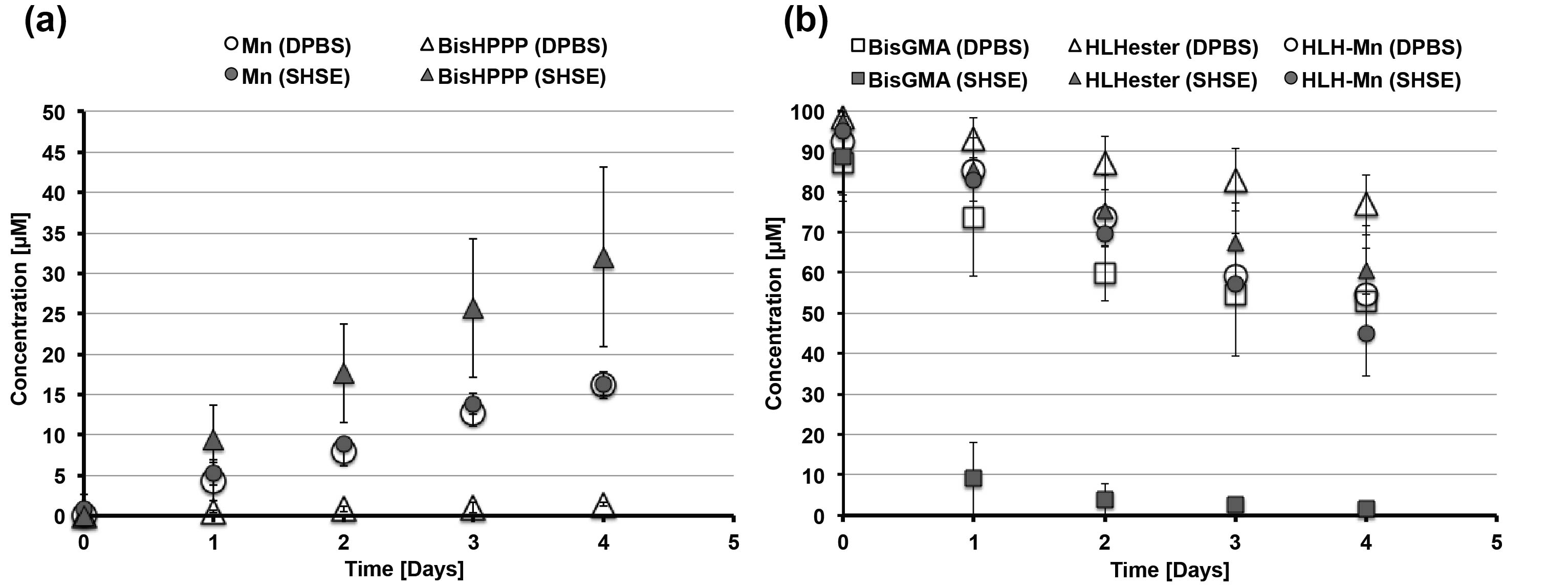
Figure 2. Degradation (a) by-products MN and BisHPPP released and (b) remaining monomer in solution, under buffer (DPBS) or enzyme conditions.
Conclusion: HLH-MN, which polymerizes with a high degree of cross-linking, is more hydrolytically stable in comparison to BisGMA and releases MN upon degradation. On-going work is assessing the efficacy of the drug release for the combination systems and determining an optimal formulation of the two drug monomer adhesive system with respect to mechanical, chemical and biological stability.
The authors would like to thank Dr. Meilin Yang for his scientific input and expertise in chemistry; NSERC CGS and NSERC Discovery Grant #360520
References:
[1] Heintze and Rousson, J Adhes Dent 2012; 14: 407
[2] Windley et al., J Endo 2005; 31: 439-43
[3] Bush, Clin Microbiol Rev 1998; 1:109-23
[4] Delaviz et al., 2015 Society for Biomaterials Annual Meeting and Exposition: Driving Biomaterial Innovation and the Race to Translation, Charlotte, NC, USA (Abstract #103)
[5] Jaffer et al., Biomaterials 2002; 32: 1707
[6] Kermanshahi et al., J Dent Res 2010; 89(9): 996-1001
[7] Santerre et al., J Dent Res 1999; 78(8): 1459-68