Introduction: Despite their popularity[1], resin composites have limited longevity in the oral cavity due to chemical hydrolysis that is believed to be facilitated in part by bacterial hydrolases within the margin of the tooth and restorative material [2][3]. To overcome this challenge, a pro-biochemical approach is being investigated where a drug monomer (DM) is introduced into resin adhesives, enabling the release of antimicrobial agents if the materials are degraded by salivary enzymes. The objective of this study was to assess the degradation of a formulated adhesive with 15wt% DM, and compare it to a control (Bis-Hema) containing the commercial monomers Bisphenol A glycidyl methacrylate (BisGMA) and 2-hydroxyethyl methacrylate (HEMA).
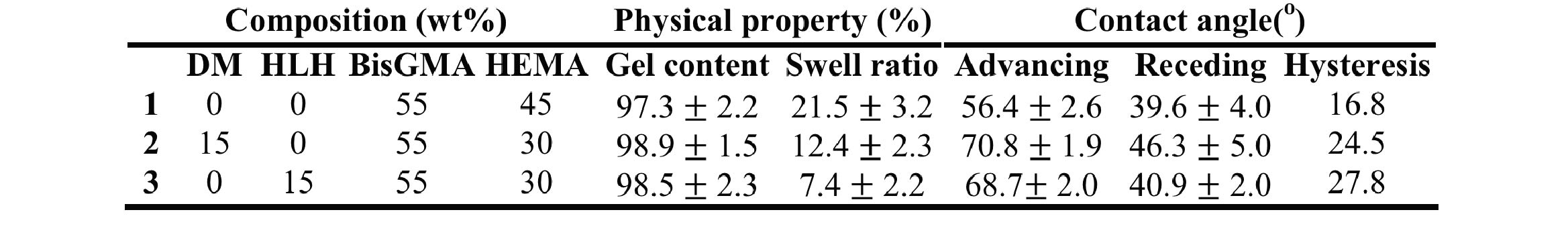
Methods: The antibiotic ciprofloxacin (CF) is chemically coupled to the backbone of a di-vinyl lysine monomer, referred to as HLH. The DM is synthesized by covalently coupling a CF/polyethylene glycol (PEG) complex via a pendant acid group on HLH[2]. Adhesive samples (2mm D, 1mm H) were formulated and light cured (1 min/side) in Teflon moulds using 0.5wt% camphorquinone and 1wt% dimethylamino-ethyl methacrylate as initiator system (Tab 1). Gel content (in dichloromethane) and swell ratio (in water) were determined (n=5) after 48 hrs of incubation. Contact angles (n=15, repeated 3 times) were measured by means of a goniometer using deionized water (Tab 1). Biodegradation studies (n=9, repeated 3 times) were performed by by incubating with either DPBS or enzyme solution (containing cholesterol esterase activity and pseudocholinesterase activity), for 28 days[3]. Enzyme activity was maintained by daily replenishment following previously established protocols[3],[4]. Degradation by-products were isolated by high performance liquid chromatography (HPLC).
Table 1. Summarizing composition, physical property and contact angle of the three formulations (1:BisHEMA, 2:DM, 3:HLH) used in the biodegradation study;±SD.
Results and Discussion: DM is about ~50x more hydrolytically stable than BisGMA[2], however the addition of the DM increased the amount of BisHPPP (terminal degradation by-product of BisGMA) released in DPBS (Fig 1a). In the presence of enzyme, there is no significant difference in the amount of BisHPPP released between the control and the DM containing formulation, but degradation is 3x higher than with DPBS (Fig 1b). Interestingly, the non-PEG precursor (i.e. HLH formulation) significantly enhanced stability (i.e. lower BisHPPP), suggesting that the lysine chemistry promotes stability. The HLH formulation has the lowest water swell ratio (Tab 1), which would also predispose it to less hydrolysis. There was no significant difference in the CF released for buffer vs enzyme (Fig 1c), suggesting that the enzyme has limited access to the pseudo-amide linkage, possibly due to steric hindrance from the bulky pendent structure of CF[5],[6].
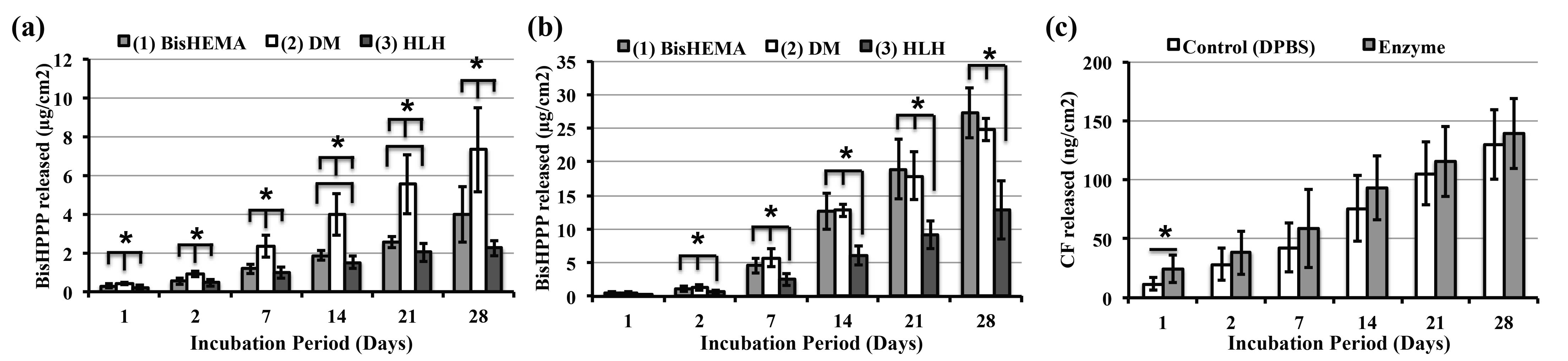
Figure 1. Terminal BisHPPP released under (a) buffer and (b) enzyme condition. (c) Terminal CF released from DM formulation for buffer and enzyme condition. Values significantly different are labeled as * (p<0.05), ±SD.
Conclusion: While the HLH component contributes to stability of the adhesive, the PEG element enables CF release via simple hydrolysis when BisGMA undergoes degradation in an enzymatic environment. On-going work is investigating the efficacy of the released drug in the presence of streptococcus mutans.
NSERC CGS and NSERC Discovery Grant #360520
References:
[1] Heintze and Rousson, J Adhes Dent 2012; 14: 407
[2] Jaffer et al., Biomaterials 2002; 32: 1707
[3] Delaviz et al., 2015 Society for Biomaterials Annual Meeting and Exposition: Driving Biomaterial Innovation and the Race to Translation, Charlotte, NC, USA (Abstract #103)
[4] Kermanshahi et al., J Dent Res 2010; 89(9): 996-1001
[5] Finer and Santerre, J Biomed Mater Res 2004; 69A, 233-246
[6] Finer and Santerre, J Dent Res 2004; 83:22-26