Introduction: Most of current cardiovascular biomaterials are non-degradable. Their permanent presence in human body can cause severe long-term complications[1]-[6]. Despite of the success of drug-eluting stent (DES) in suppressing short-term complications, the long-term efficacy of DES is questioned due to negative effects of its drugs on endothelium restoration[1],[3],[6],[7]. An emerging strategy to address the problems of permanent coronary stent is the use of bioresorbable cardiovascular scaffold (BCS). BCS is made of bioresorbable materials that will be resorbed by the body over time. The absence of implant materials avoids chronic foreign body responses and rules out the limit to vasomotor activities. Although bioresorbable polymers were the first category of materials to be tested for making BCS[8], their inflammatory degradation products and difficult implantation procedure compromised their effectiveness[8],[9]. Magnesium (Mg) metals, on the other hand, have great potentials due to their innate degradability and biocompatibility. Nevertheless, the rapid degradation of pure Mg is not suitable for this application. Pure Mg is unable to maintain necessary mechanical strength before the completion of endothelialization. Moreover, human trial (BIOSOLVE-I) found that Mg-based BCS has large recoil caused by in-stent thrombosis and neointimal hyperplasia[10]. The presented work uses bioresorbable aliphatic polyester as coating materials for high purity Mg. The polymer coatings are expected to reduce Mg degradation rate. It is also anticipated that the polymer coatings will promote endothelialization. A promoted endothelium restoration can minimize thrombus formation and neointimal hyperplasia. This study investigates how materials affect human umbilical vein endothelial cells (HUVECs). By determining HUVECs adhesion after in vitro culture with materials, the potentials of polymer-coated Mg as bioresorbable cardiovascular material will be revealed.
Materials and Methods: High purity Mg (99.9% purity) was coated with polymers by spin coating. Four polymers were selected as coating materials for this project: poly(L-lactic acid) (PLLA), poly(lactic-co-glycolic acid) (PLGA, including PLGA (90:10) and PLGA (50:50)), and polycaprolactone (PCL). HUVECs were incubated with materials for 24h. HUVECs adhesion was characterized using fluorescence images of cells.
Results and Discussion: The results of material characterization showed that intact polymer coatings were successfully prepared on pure Mg. In vitro cell assay indicated that PLGA (50:50)-coated Mg has greatly promoted HUVEC adhesion on its surface, which is shown in figure 1.
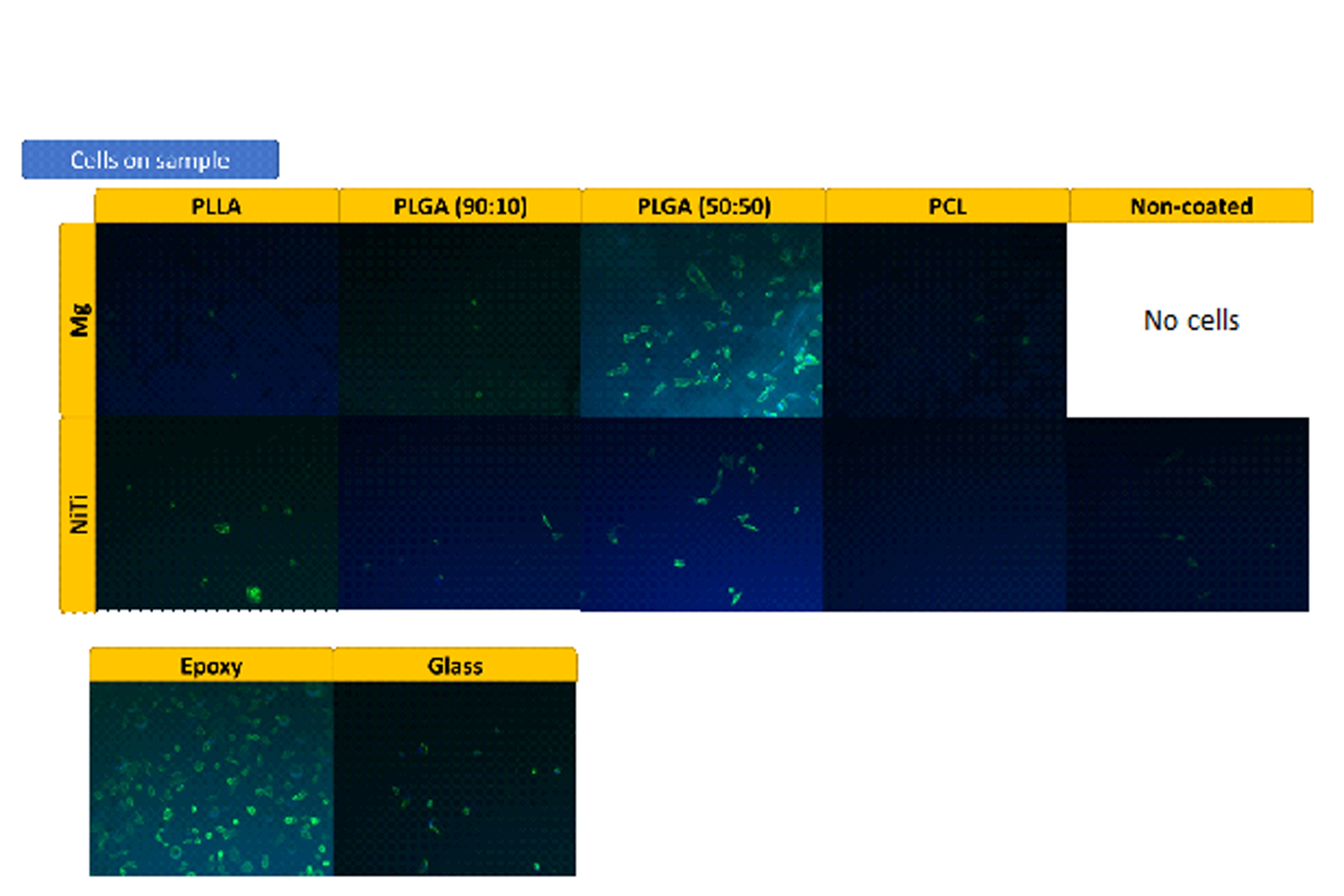
Figure 1: Mg2+ is potentially beneficial for HUVECs adhesion. Unfortunately, HUVECs do not attach to the bare Mg surface due to the fast degradation. Therefore, PLGA (50:50) is an ideal coating material because 1) it permits the release of beneficial Mg2+, 2) it provides HUVECs a relatively stable surface to attach to at the early stage. PLGA (50:50)-coated Mg is promising to be used as a bioresorbable cardiovascular material.
Central Facility for Advanced Microscopy and Microanalysis (CFAMM), University of California, Riverside; Center for Nanoscale Science & Engineering, University of California, Riverside; American Heart Association (AHA) for financial support
References:
[1] Serruys, P.W., M.J. Kutryk, and A.T. Ong, Coronary-artery stents. New England Journal of Medicine, 2006. 354(5): p. 483-495.
[2] Newsome, L.T., et al., A protocol for the perioperative management of patients with intracoronary drug-eluting stents. Anesthesia Patient Safety Foundation. APSF Newsletter, 2006. 4: p. 81-2.
[3] Lüscher, T.F., et al., Drug-eluting stent and coronary thrombosis biological mechanisms and clinical implications. Circulation, 2007. 115(8): p. 1051-1058.
[4] Carpenter, A.W. and M.H. Schoenfisch, Nitric oxide release: Part II. Therapeutic applications. Chemical Society Reviews, 2012. 41(10): p. 3742-3752.
[5] Farb, A., et al., Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation, 2003. 108(14): p. 1701-1706.
[6] O’Brien, B. and W. Carroll, The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: a review. Acta biomaterialia, 2009. 5(4): p. 945-958.
[7] Lagerqvist, B., et al., Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. New England Journal of Medicine, 2007. 356(10): p. 1009-1019.
[8] Tamai, H., et al., Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation, 2000. 102(4): p. 399-404.
[9] Van Der Giessen, W.J., et al., Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation, 1996. 94(7): p. 1690-1697.
[10] Haude, M., et al., Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. The Lancet, 2013. 381(9869): p. 836-844.