Immobilization of cell adhesive peptide on polymeric and metallic surfaces using dopaquinone produced by the direct oxidation of tyrosine residue
-
1
Kansai University, Department of Chemistry and Materials Engineering, Japan
-
2
National Cerebral and Cardiovascular Center Research Institute, Department of Biomedical Engineering, Japan
Introduction: The compatibility with cell or tissue is necessary in prosthesis and scaffolds for tissue engineering. Since biological activities are not inherent in the most of biomaterials, immobilization of biologically active peptides which were isolated from extracellular matrix proteins is used as one of the powerful strategies for the design and functionalization of biomaterial surface. For example, biomimetic surface which is immobilized with integrin binding motif such as RGDS peptide can provide cellular adhesiveness. However, the method of peptide immobilization was restricted. Typically, peptides were immobilized through the previously-introduced reactive group on the material surface. P. B. Messersmith et al. have reported the modification of biomaterial surfaces using 3,4-dihydroxy-L-phenylalanine (DOPA), which is known for its contribution to the strong adhesive properties of marine mussel proteins[1]. This method has some restrictions, for example, catechol hydroxyl groups must be capped with special protecting groups during the synthesis of peptides containing DOPA residues. In this report, we immobilized endothelial cell adhesive REDV peptide onto a several kinds of polymer and metal surfaces using the direct oxidation from Tyr residue to dopaquinone using hydrogen peroxide and copper (II) chloride catalyst[2].
Materials and Methods: REDV peptide containing a Tyr residue (Ac-TGGGREDV) was synthesized by typical Fmoc solid phase procedure. Substrates such as glass, tissue culture polystyrene (TCPS), polyester (Cell Desk, Sumitomo Bakelite Co., Ltd.) (PESr), tissue culture poly (vinyl chloride) (PVC), expanded poly (tetrafluoroethylene) (ePTFE), poly (L-lactic acid) (Mw=160000) (PLLA) and 316L stainless steel (SS316L) were immersed into an aqueous solution of the peptide, and then H2O2 and CuCl2 were added and incubated for 24 hours. After REDV immobilization, surfaces were analyzed by the water contact angle measurement and X-ray photoelectron spectroscopy (XPS). The adhesive property of human umbilical vein endothelial cell (HUVEC) on REDV- immobilized surfaces was evaluated in vitro.
Results and Discussion: The water contact angle was decreased and a N1s peak assigned to the peptide was detected in XPS spectra in all substrates after peptide immobilization. These behaviors were not exhibited in the absence of CuCl2 and H2O2 resulting in the immobilization of REDV peptide by Tyr oxidation. The adhesion and spreading of HUVECs were improved in all substrates by REDV immobilization, but the number of adhesive HUVEC was affected by the substrates (Figure 1). The low adhesion of HUVECs on peptide-immobilized PVC, ePTFE and PLLA was because of a low amount of immobilized REDV.
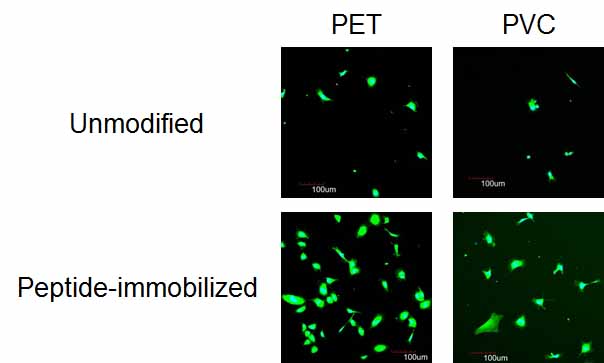
Figure 1. HUVEC adhesion on unmodified and REDV-immobilized PET and PVC surfaces in the presence of FBS and growth factors.
Conclusion: We successfully developed a simple and direct method for immobilizing cell adhesive peptides onto polymer and metal material surfaces without losing biological function. Therefore, we expect this method could be applied for biofunctionalization of medical devices composed of multiple materials.
References:
[1] Lee H. et al., . Science. 2007: 318: 426-430.
[2] Kakinoki S. et al., Bioconj. Chem. 2015: 26: 639-644
Keywords:
Cell Adhesion,
Surface modification,
Bioactive molecule,
bioactive interface
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Bioconjugates
Citation:
Kakinoki
S,
Mahara
A,
Yamaoka
T and
Hirano
Y
(2016). Immobilization of cell adhesive peptide on polymeric and metallic surfaces using dopaquinone produced by the direct oxidation of tyrosine residue.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01890
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Sachiro Kakinoki, Kansai University, Department of Chemistry and Materials Engineering, Suita, Osaka, Japan, Email1
Dr. Atsushi Mahara, National Cerebral and Cardiovascular Center Research Institute, Department of Biomedical Engineering, Suita, Osaka, Japan, mahara@ncvc.go.jp
Dr. Tetsuji Yamaoka, National Cerebral and Cardiovascular Center Research Institute, Department of Biomedical Engineering, Suita, Osaka, Japan, yamtet@ncvc.go.jp
Dr. Yoshiaki Hirano, Kansai University, Department of Chemistry and Materials Engineering, Suita, Osaka, Japan, yhirano@kansai-u.ac.jp