A synthetic heparin antidote with negligible effect on fibrin(ogen), clotting and clot morphology
-
1
University of British Columbia, Pathology and laboratory Medicine, Canada
-
2
University of British Columbia, Department of Immunolgy and Microbiology, Canada
-
3
University of British Columbia, Department of Chemical and Biological Engineering, Canada
-
4
University of British Columbia, Chemistry, Canada
Background and Objective:
Heparin anticoagulants are used to treat thrombosis; the leading cause of deaths worldwide. However, anticoagulation associated haemorrhage is a major concern. Hence, antidote is required to counteract anticoagulation and to restore hemostasis. Protamine sulphate (PS), a cationic polypeptide is the only clinically approved antidote for unfractionated heparin. PS is unable to completely reverse low molecular weight heparins and fondaparinux due to its low binding affinity to these drugs. In addition, PS exhibits non-specific interaction with clotting proteins such as fibrinogen to form aggregates and alters fibrin clot morphology. This often leads to hematological complications. To address these challenges, we developed a synthetic universal heparin reversal agent (UHRA) with high binding affinity to heparins, thereby completely reversing the activity of all clinically available parenteral anticoagulants1.This study aims to demonstrate and corroborate the nontoxic nature of UHRA by assessing its influence on fibrinogen, clotting, fibrin/blood clot architecture and stability.
Methods:
UHRA was developed by incorporating tertiary amine based heparin binding groups on a dendritic hyperbranched polyglycerol scaffold and capping it with methoxy polyethylene glycol chains. Recalcification and tissue factor (TF) initiated plasma clotting assays was performed to understand the impact of UHRA on clotting system. The interaction of UHRA on fibrinogen was investigated by fibrin polymerization assay and by spectroscopic analysis (fluorescence and circular dichroism (CD)). The influence of UHRA on fibrin/blood clot architecture was evaluated by scanning electron microscopy (SEM). Confocal microscopy was used to investigate the incorporation of Alexa-488 tagged UHRA into fibrin clots. The lysis of TF-induced plasma clot containing UHRA or PS exposed to exogenous tissue plasminogen activator (t-PA) was studied by turbidimetric assay.
Results and discussion:
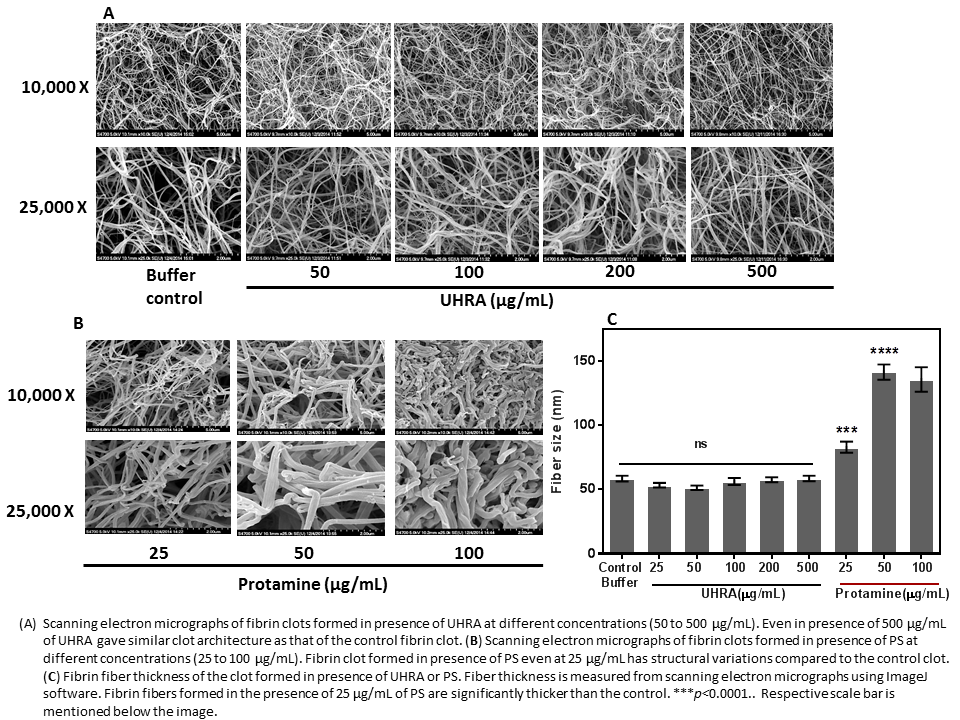
Clotting assays revealed that UHRA did not alter clotting parameters (lag time and maximum absorbance) compared to PS. This suggests that UHRA has no effect on clotting cascade. Unlike protamine, the fibrinogen aggregation and fibrin polymerization assay was not influenced by UHRA over a broad range of concentrations from 0.05 mg/mL to 1 mg/mL. Tryptophan fluorescence quenching and fibrinogen secondary structure measurements, corroborates that UHRA is not interacting with fibrinogen. However, PS and other synthetic cationic polymers interact with fibrinogen, eliciting aggregation and conformational changes. Fibrin clots generated in presence of UHRA (even at 0.5 mg/mL) showed similar structure and fiber size remains same as normal fibrin clot (control vs UHRA 0.5 mg/mL clot, p= 0.12) (Figure 1). On the other hand, fibrin clots formed in the presence of 0.05mg/mL PS (clinical dose) increased the fiber size and changed the clot structure dramatically (control vs PS 0.05mg/mL clot, p< 0.0001).
Confocal images of fibrin clots show no incorporation of UHRA into clots, compared to PS (Figure 2). 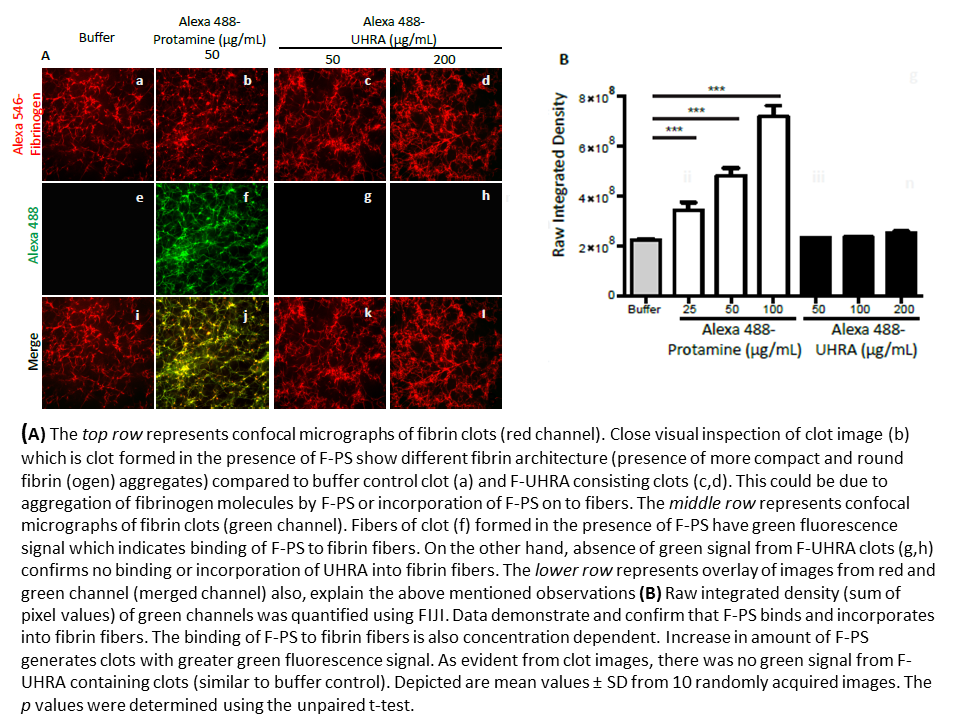
Clot lysis studies in the presence of exogenous t-PA demonstrate that UHRA did not enhance clot degradation.
Conclusion and significance:
Studies demonstrate that, UHRA, has negligible impact on fibrinogen, fibrin polymerization, clot structure, clot degradation, coagulation system and shows no binding to clot structures, revealing their excellent hemocompatibility compared to protamine. Our results support the fact that UHRA could be an ideal antidote to restore hemostasis following invasive surgical procedures and to address bleeding complications by heparin based anticoagulants.
[1]
Canadian Institutes of Health Research (CIHR); Natural Science and Engineering Research Council (NSERC)
References:
[1] Shenoi et .al, Affinity-based design of a synthetic universal reversal agent for heparin anticoagulants. Sci Transl Med 6, 260ra150 (2014).
Keywords:
nanoparticle,
Bioactivity,
biomacromolecule,
hemocompatiblility
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Biomaterials for cardiovascular applications, vascular grafts and embolic devices
Citation:
Kalathottukaren
M,
Lai
BF,
Abraham
L,
Rosell
F,
Pryzdial
EL,
Haynes
CA and
Kizhakkedathu
JN
(2016). A synthetic heparin antidote with negligible effect on fibrin(ogen), clotting and clot morphology.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01836
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Manu Thomas Kalathottukaren, University of British Columbia, Pathology and laboratory Medicine, Vancouver, BC, Canada, Email1
Dr. Benjamin F Lai, University of British Columbia, Pathology and laboratory Medicine, Vancouver, BC, Canada, Email2
Dr. Libin Abraham, University of British Columbia, Department of Immunolgy and Microbiology, Vancouver, BC, Canada, Email3
Dr. Fredrico Rosell, University of British Columbia, Pathology and laboratory Medicine, Vancouver, BC, Canada, Email4
Dr. Charles A Haynes, University of British Columbia, Department of Chemical and Biological Engineering, Vancouver, BC, Canada, Email5
Dr. Jayachandran N Kizhakkedathu, University of British Columbia, Pathology and laboratory Medicine, Vancouver, BC, Canada, jay@pathology.ubc.ca