Introduction:Porous scaffold plays pivotal roles in bone tissue engineering. The porous structure, including average pore size, porosity and interconnectivity determine not only the mechanical properties but also their biological performance of porous scaffolding materials [1]. Pore interconnectivity affects the biological response of a scaffold by influencing the transport of nutrients to and metabolites out from the tissue-engineered constructs, homogeneous cell seeding and migration, and tissue ingrowth [2]. The axially aligned channels within scaffolds build an anisotropic architecture, which would improve interconnectivity of the scaffold and help the infiltration of cells and tissue into the scaffolds [3]. Herein, a nano-hydroxyapatite/polyamide66 (n-HA/PA66) lotus-type (anisotropic) scaffold with axially aligned channels was prepared to enhance pore interconnectivity and the performance both in vitro and in vivo was assessed.
Materials and Methods: The n-HA/PA66 slurry was prepared using the coprecipitation method in ethanol [4]. Stainless steel wires of 300 μm diameter were coated with the n-HA/PA66 slurry. The coated wires were bundled in heat shrink tubing, then heated in an oven to activate the heat shrink tubing and to induce the evaporation of the ethanol and solidify the porous structure. The tubing and wires were then carefully removed to reveal the axially aligned channels in the scaffold. Porous n-HA/PA66 isotropic scaffolds produced by the thermally induced phase separation (TIPS) technique were used as a control. The architecture, porosity, mechanical properties of fabricated scaffolds were investigated. The biological performance including in vitro cell infiltration and in vivo bone tissue regeneration and ingrowth into the inner parts of the fabricated scaffolds by using repairing critical 15-mm segmental bone defects in the radius and cranial defect animal models respectively in rabbit was also evaluated.
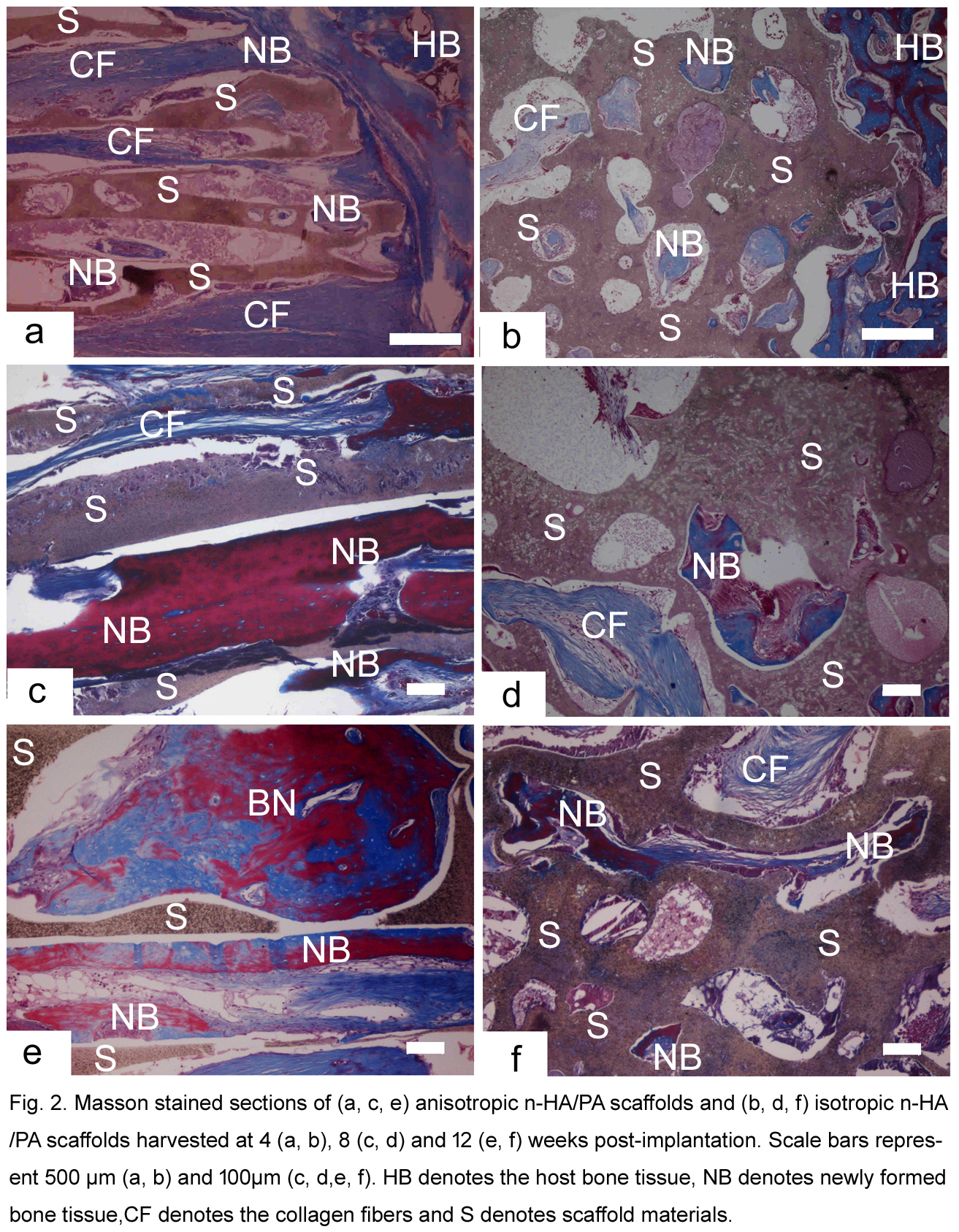
Results and Discussion: The n-HA/PA66 lotus-type scaffolds with axially aligned channels in 300μm diameter were controllably prepared to enhance pore interconnectivity throughout the scaffold (Figure 1). Lotus-type scaffolds with axially aligned channels had better mechanical properties and a higher porosity (86.37%) than isotropic scaffolds produced by TIPS. The channels in the lotus-type scaffolds provided cells with passageways to the scaffold center and thus facilitated cell attachment and proliferation inside the scaffolds. In vivo studies showed that the anisotropic scaffolds could better facilitate new bone ingrowth into the inner pores of the scaffold compared to the isotropic scaffolds (Figure 2). The anisotropic scaffolds also had improved vascular invasion into their inner parts, increasing the supply of oxygen and nutrients to the cells and thus facilitating revascularization and bone ingrowth. Enhanced cell and tissue penetration to the scaffold center was observed in the anisotropic scaffolds both in vitro and in vivo, indicating the axially aligned channels positively influenced cell and tissue infiltration.
Conclusion: In vitro and in vivo data indicate cell penetration and tissue infiltration were enhanced in the controllably fabricated anisotropic scaffold and should serve to reduce the incidence of a necrotic core.

The authors acknowledge funding support from China 863 project (2013AA032203), Outstanding Young Scholar Fund of Sichuan University (2014SCU04A20)
References:
[1] Zhang Y, Yao NJ, Wang F, Li WD, Jiang SX . A novel in situ self foaming method for the synthesis of porous calcium metaphosphate biomaterials. RSC ADVANCES 2014; 4 (104): 60007-60016.
[2] Jonesa AC, Arnsa CH, Hutmacher DW, Milthorpec BK, Shepparda AP, Knackstedt MA. The correlation of pore morphology, interconnectivity and physical properties of 3D scaffolds with bone ingrowth. Biomaterials 2010; 31(25):6494-501.
[3] Silva M, Cyster, LA, Barry JJA, Yang, XB, Oreffod ROC, Grant DM, Scotchford C.A, Howdlea SM, Shakesheffc KM, Rose FRAJ. The effect of anisotropic architecture on cell and tissue infiltration into tissue engineering scaffolds. Biomaterials 2006; 27: 5909-5917.
[4] Wang H, Zuo Y, Zou Q, Cheng L, Huang D, Wang L, Li Y. Nano-hydroxyapatite/polyamide66 composite tissue-engineering scaffolds with anisotropy in morphology and mechanical behaviors. J Polym Sci, Part A-1: Polym Chem 2009; 47: 658-669.