Hollow mesoporous carbon used for oral bioavailability improvement of insoluble drug: a preliminarily study both in vitro and in vivo
-
1
Shenyang Pharmaceutical University, Department of Pharmaceutics, School of Pharmacy, China
-
2
Shenyang Pharmaceutical University, School of Traditional Chinese Materia Medica, China
-
3
Shenyang Pharmaceutical University, School of Medical Devices, China
Introduction: Recently, mesoporous carbon nanoparticles have been applied to improve the oral bioavailability of insoluble drugs. On the one hand, the large surface area and numerous mesopores of carbon materials help drugs to disperse and accelerate their dissolution rate. On the other hand, carbon have strong adsorption capacity, so they may adhere to gastrointestinal mucosa and enlarge the absorption time of the loaded drugs. Although carbon has a lot of advantages, the low drug loading and premature release usually hindered its use. The traditional solution was to cover the carbon vehicles with polymers, however, the synthesis process was always complicated. In order to notably increase the drug loading and easily adjust the drug release behavior, we designed a structure-controllable hollow mesoporous carbon (HMC) to enhance the oral bioavailability of the loaded drugs. In addition, to preliminarily study the in vivo process of HMC, we labeled HMC with a kind of rare-earth ion Eu3+.
Methods and Results: During synthesis, tetraethoxysilane as silica source was added twice, and hexadecyltrimethylammonium bromide with different amount was used to adjust the channel length. Oxalic acid and furfuryl alcohol as carbon source were extruded into template’s crevices. After heating, silica was removed by hydrofluoric acid, and then HMC was obtained. Followed by carboxylating with sulfuric acid (H2SO4), HMC was labeled with a complex of Eu3+/Gd3+ EDTA at pH 6.4.
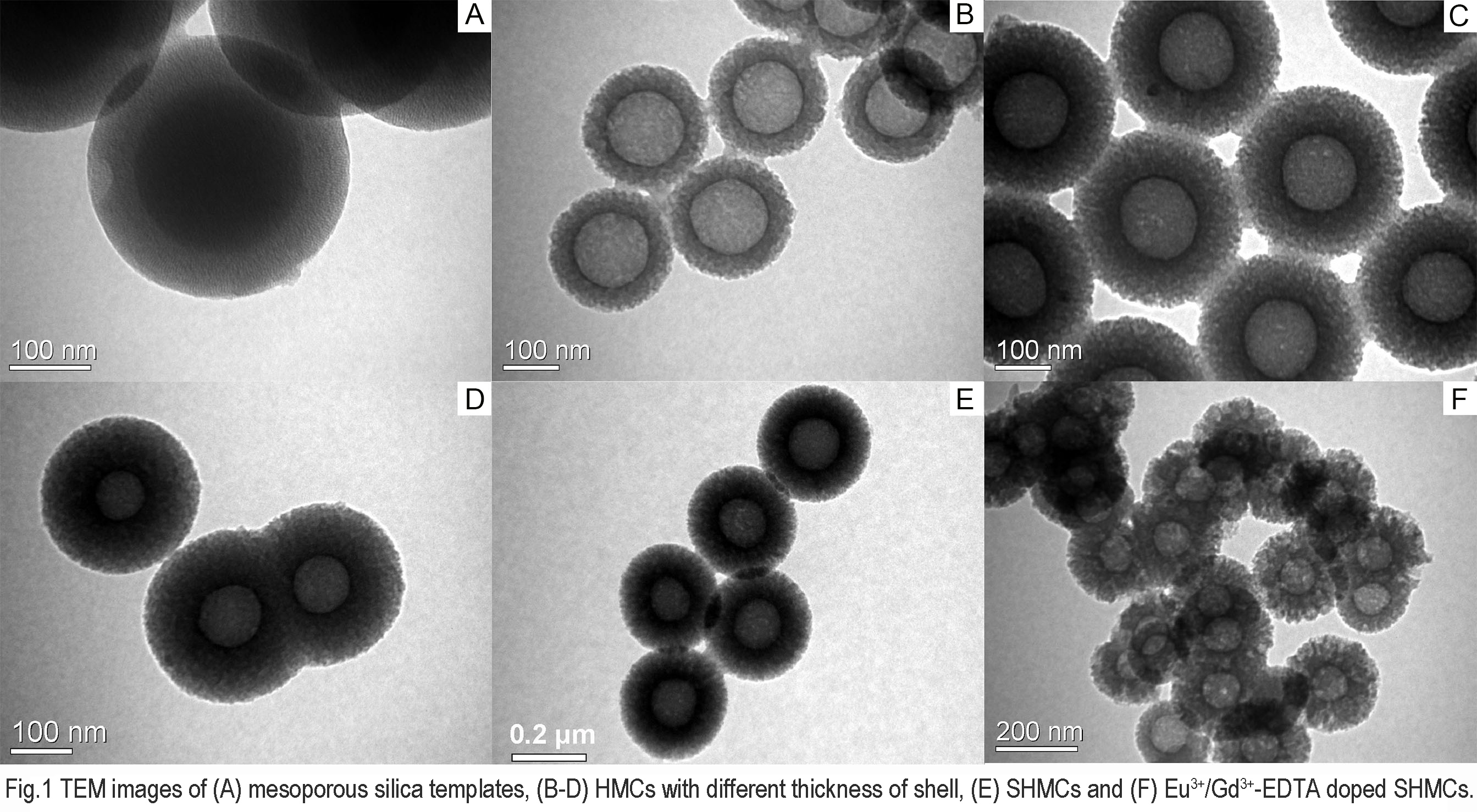
Physicochemical properties of the composites were characterized by transmission electron microscope (TEM), Fourier transform infrared spectroscopy (FT-IR), and photoluminescence (PL) spectra test prior to studying their effects on in vitro/in vivo drug release behavior and bio-distribution. Results are shown in Fig. 1 and Fig. 2. As a result, the thickness of the shell was adjusted from 70 to 130 nm and the maximum drug loading was as high as 73.6%. The model drug carvedilol (CAR) showed a sustained-release behavior compared to CAR capsules, and the dissolution rate was slower as the shells getting thicker. AUC0-48h and Tmax of CAR was 2.2 and 6.5 fold enlarged after being loaded in SHMC. Bio-distribution tests showed that the vehicles had a 5 days’ residence in gastrointestinal tract, and they were mainly delivered to liver and kidney. Gastrointestinal irritation tests ensured the feasibility of long retention time, and the rare aggregation in other organs or tissues decreased the side effect of the drugs.
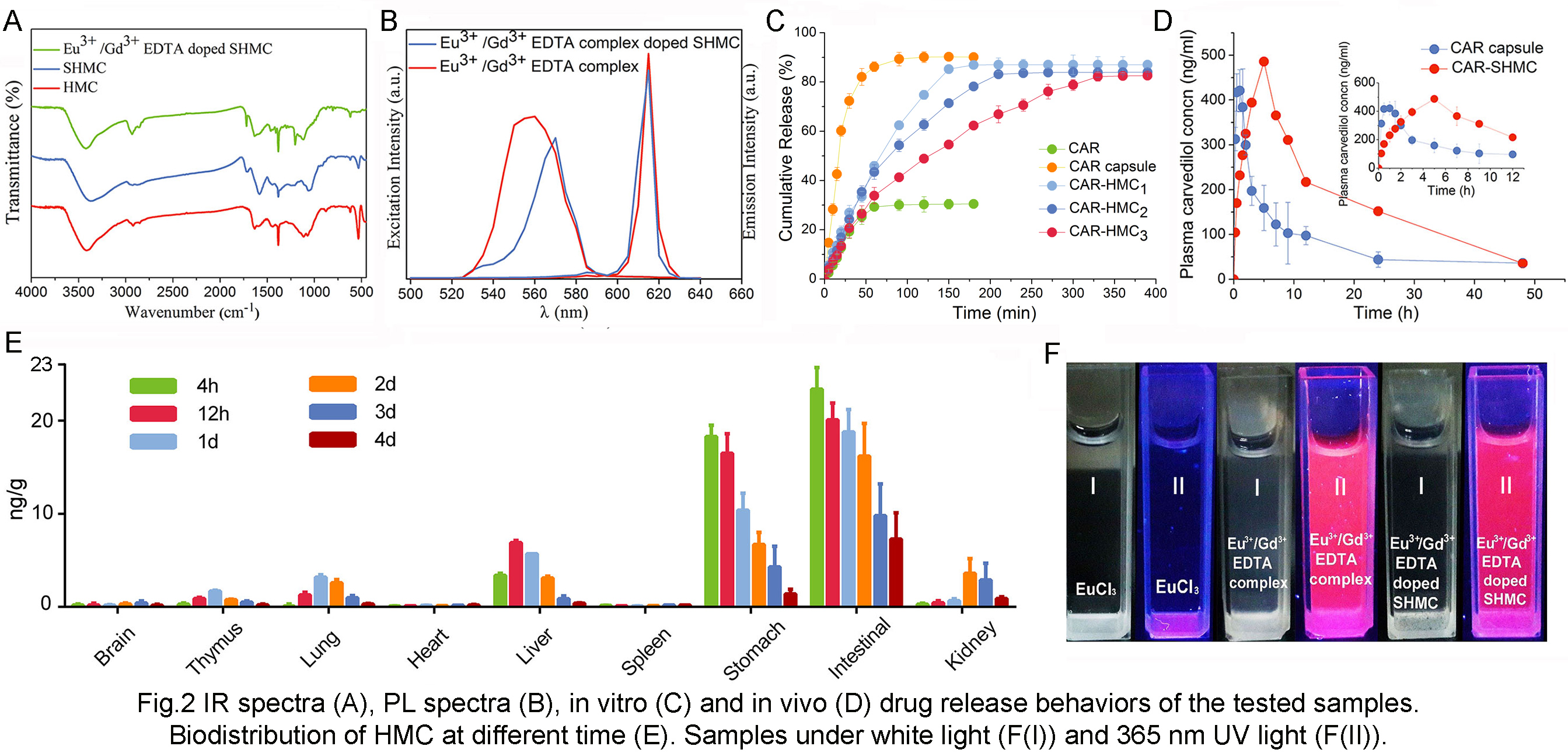
Conclusions: In conclusion, we established a Eu3+/Gd3+-EDTA doped structure-controllable HMC. A high drug loading and a sustained release behavior were obtained. By bio-distribution test, a long residence time in gastrointestinal tract was clearly observed. All these characters make HMC an ideal carrier to improve the oral bioavailability of insoluble drugs.
This work was supported by National Basic Research Program of China (973 Program, No.2015CB932100) and Key Laboratory Basic Research Program of the Education Department of Liaoning Province (No. LZ2015068)
Keywords:
Drug delivery,
biomaterial,
Nano/micro particle
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials for therapeutic delivery
Citation:
Liu
J,
Sun
C,
Hou
B,
Zhao
Q,
Gao
Y,
Wang
Y and
Wang
S
(2016). Hollow mesoporous carbon used for oral bioavailability improvement of insoluble drug: a preliminarily study both in vitro and in vivo.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01776
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Jia Liu, Shenyang Pharmaceutical University, Department of Pharmaceutics, School of Pharmacy, Shenyang, China, Email1
Dr. Changshan Sun, Shenyang Pharmaceutical University, Department of Pharmaceutics, School of Pharmacy, Shenyang, China, Email2
Dr. Bailing Hou, Shenyang Pharmaceutical University, School of Traditional Chinese Materia Medica, Shenyang, China, Email3
Dr. Ying Wang, Shenyang Pharmaceutical University, Department of Pharmaceutics, School of Pharmacy, Shenyang, China, Email4