Introduction: Polymeric micelles have been heavily investigated for their use as vehicles to deliver poorly soluble, potent chemotherapeutics[1]. While these systems have shown clinical promise, poor stability and low drug encapsulation have hindered the translation of many formulations.
Our lab has designed a unique biodegradable polymer comprised of a hydrophobic copolymer backbone and a hydrophilic graft, poly(D,L-lactide-co-2-methyl-2-carboxytrimethylene carbonate)-graft-poly(ethylene glycol) (P(LA-co-TMCC)-g-PEG)[2]. This polymer self-assembles in water to form polymeric micelles with low dispersity.
The free carboxylates on the TMCC of the backbone serve as a functional handle to chemically modify the polymer and tune its properties for drug delivery. Specifically, modifications of the hydrophobic core facilitate specific interactions with the drug to provide greater encapsulation without jeopardizing stability.
Materials and Methods: P(TMCC-co-LA)-g-PEG was synthesized as previously described[3]. Hydrophobic modifications to the backbone were done by conjugating moieties, such as docetaxel or peptides, to the carboxylic acid on the backbone either directly, using a Steglich esterification, or using a Michael-addition by modifying the carboxylic acid with 3,3’-dithiobis(propionic hydrazide) and reducing to produce a free thiol[4]. Docetaxel loaded polymeric micelles were synthesized using a dialysis self-assembly procedure and characterized using a Malvern Zetasizer Nano ZS. The cytotoxicity of empty and drug-loaded polymeric micelles was evaluated using the Presto-Blue assay on SKBR-3 cells. Kinetic stability in 20% Fetal Bovine Serum (FBS) was determined using size exclusion chromatography of particles incubated at various time points. Micelle peak areas were monitored by absorbance to evaluate dissociation of polymeric micelles, and drug loading over time was assessed by HPLC-MS/MS. The efficacy of docetaxel loaded polymeric micelles will be assessed in an orthotopic model of breast cancer in nod-scid gamma mice.
Results and Discussion: Polymers were successfully synthesized with benzyl groups (PBn), conjugated docetaxel (PDTX), or a peptide from docetaxel’s native binding site on the microtubule (PTBP). As shown in Figure 1, all modifications increased loading significantly compared to unmodified polymers. Impressively, PTBP formed micelles with loadings five times higher than the control, suggesting a high affinity of the drug for the peptide-polymer core. This specific interaction was confirmed by comparing to a scrambled peptide sequence that showed only a modest increase in drug loading similar to drug conjugated polymers.
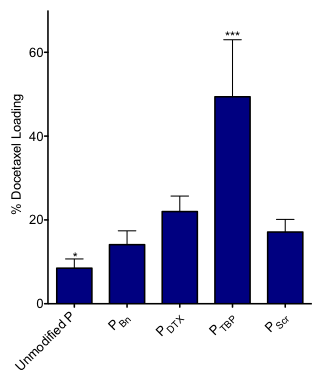
Figure 1. Percent drug loading relative to the mass of hydrophobic backbone. (n = 4-6, * p<0.05, ***p<0.005).
Taxol binding peptide modified polymers showed good cytocompatibility with SKBR-3 cells, and when loaded with drug showed similar efficacy in vitro to free drug controls. Importantly, this peptide modification does not come at the expense of kinetic stability, showing no significant differences in docetaxel release in serum conditions compared to the unmodified polymeric micelles (Figure 2).
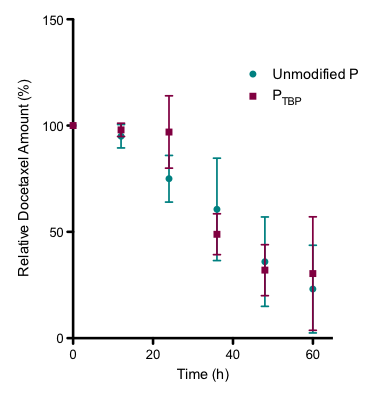
Figure 2. Serum stability of docetaxel-loaded PTBP vs. unmodified polymer over 60 h.
Conclusions: Specific hydrophobic modifications increased drug loading to up to five times that of the unmodified polymers without impeding stability in serum conditions. Our polymeric micelles represent a drug delivery system that overcomes many of the current challenges. Ongoing work includes the assessment of this system in vivo.
Natural Science and Engineering Research Council of Canada (NSERC); Canadian Institute for Health Research (CIHR)
References:
[1] Elsabahy, M. & Wooley, K. L. Chem Soc Rev 41, 2545–2561 (2012).
[2] Lu, J. & Shoichet, M. S. Macromolecules 43, 4943–4953 (2010).
[3] Logie, J., Owen, S. C., McLaughlin, C. K. & Shoichet, M. S. Chem. Mater. 26, 2847–2855 (2014).
[4] Logie, J., McLaughlin, C. K., Tam, R. Y. & Shoichet, M. S. Chem. Comm. 51, 12000–12003 (2015).