Experimental analysis of effect of electric field on mouse myoblast cells using high throughput microfluidic bioreactor
-
1
Indian Institute of Science, Centre for Biosystems Science and Engineering (BSSE), India
-
2
Indian Institute of Science, Materials Research Centre, India
-
3
Indian Institute of Science, Department of Chemical Engineering, India
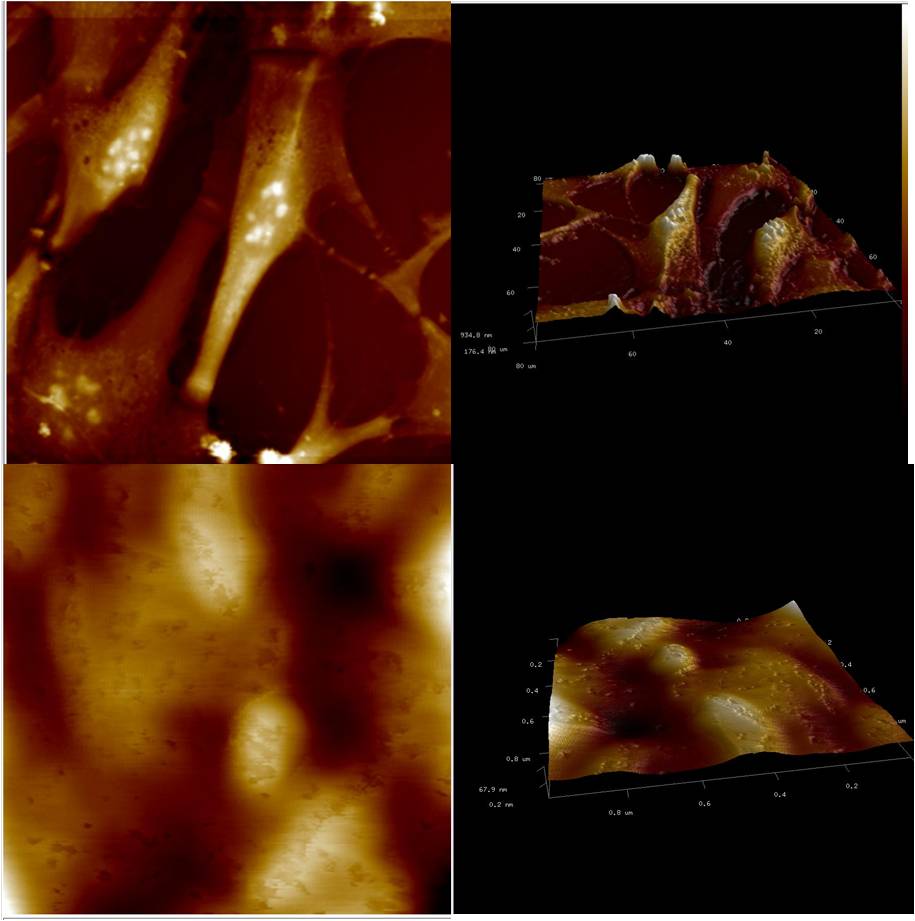
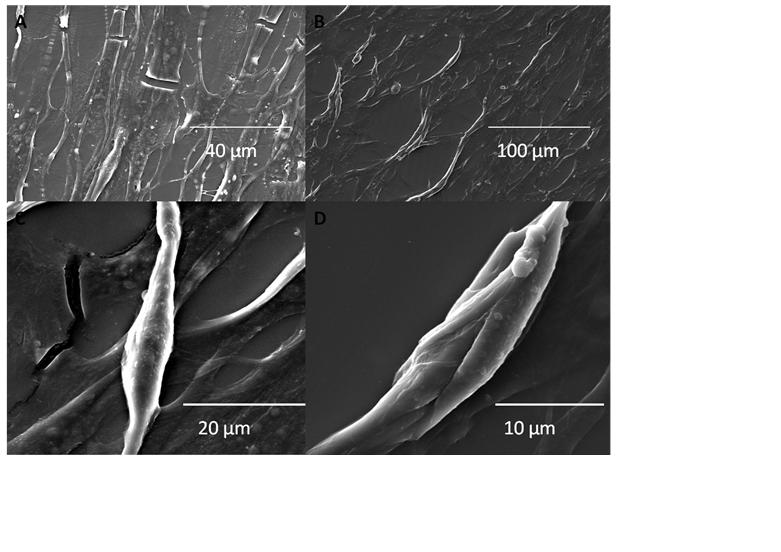
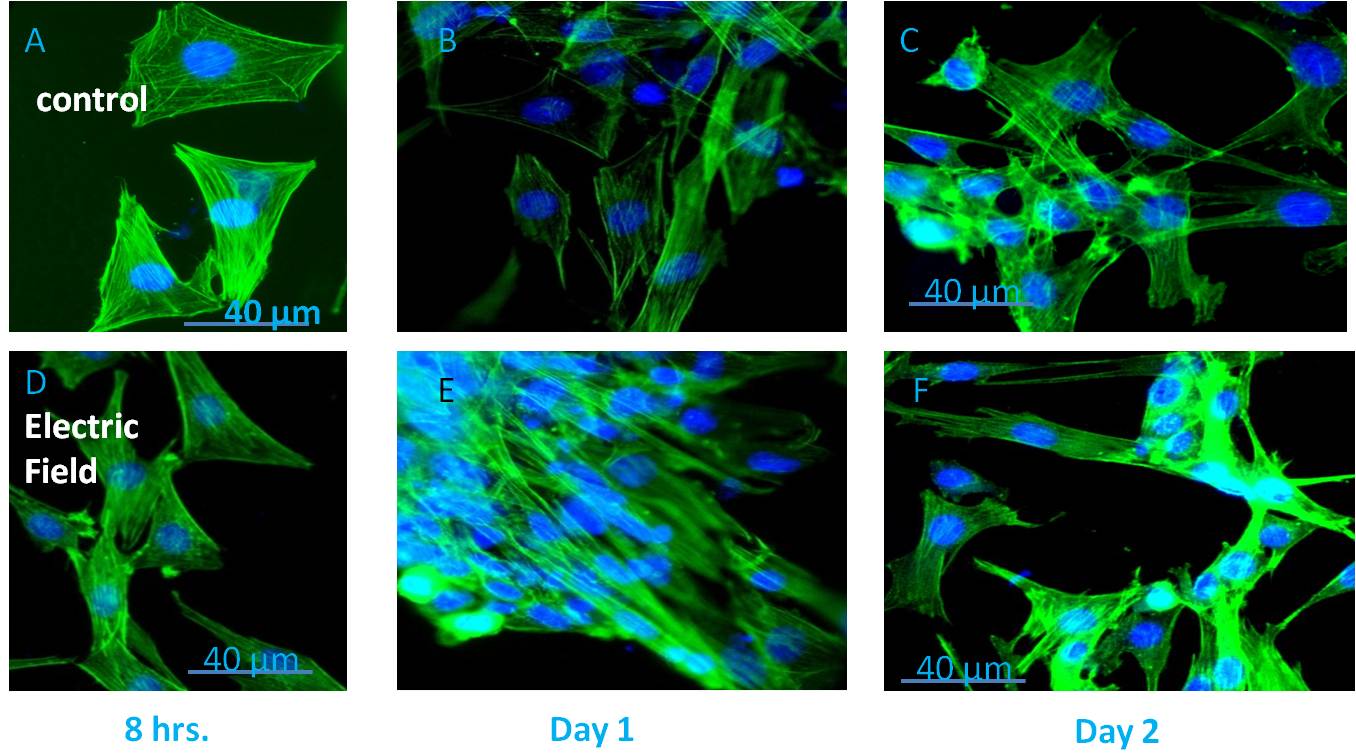
Application of electric field on excitable cells like myoblast[1] or neuroblast[2], causes cell phenotype changes which have already been investigated using conventional 2D cell culture. But this may not be a good representation of the in vivo conditions due to very high ratio of volume of culture media to the number of cells and also due to absence of the shear stress in comparison to in vivo conditions where cells continuously experience fluid flow around them. In this study, we had attempted to investigate the effect of electric field on the cells by introducing biomimetic devices that simulates the in vivo environment, using microfluidic technology. Microfluidics has already started to invade almost every corner of life sciences with the promise of more effective and efficient performance than the existing techniques with the requirement of very scanty amount of sample volume, reduction in the running cost of the experiments, high accuracy due to almost zero dead-volume and fast response time. In order to achieve an environment having close resemblance with the in vivo conditions, C2C12 (mouse myoblast) cells were cultured within the microfluidic bioreactor along with flow of culture media. Designing of this microfluidic bioreactor involved some crucial processes, including the microfluidic layout, selection of materials, fabrication process, and sterilization technique along with necessary requirements like the maintenance of sterility and biocompatibility. This microfluidic bioreactor was fabricated through photolithography by procuring a silicon based SU8 master. This SU8 master was used as a mold for device fabrication using Polydimethylsiloxane (PDMS). The C2C12 cells were then seeded inside the microfluidic channels (width of 20μm - 40μm) of the bioreactor device. By limiting both the space and nutrient for the cultured cells along with an exposure to shear stress due to flowing of fluid, this system provided a close resemblance with the in vivo scenarios. Prior exposure of mouse myoblast cells to an electric field of 12.5 V/cm for 12 hours resulted in morphological alterations with elongated nucleus, roughening of the cell suface topography, myogenesis, as revealed by immunofluorescence techniques, electron microscopy and PCR supported with biochemical assays. Overall, the results confirmed considerable morphological changes of the myoblasts, when stimulated electrically compared to the non-stimulated cells.
Prof. Bikramjit Basu; Prof. V. Kumaran
References:
[1] Thrivikraman G, Madras G, Basu B. Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials 2014 Aug;35(24):6219-35
[2] Jain S, Sharma A, Basu B. Vertical electric field stimulated neural cell functionality on porous amorphous carbon electrodes. Biomaterials 2013 Dec;34(37):9252-63 doi: 101016/jbiomaterials201308057 Epub 2013 Sep 10
Keywords:
Biomimetic,
cell phenotype,
electric,
surface topolography
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Microdevices: reproducing physiology at microscale
Citation:
Naskar
S,
Basu
B and
Kumaran
V
(2016). Experimental analysis of effect of electric field on mouse myoblast cells using high throughput microfluidic bioreactor.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01732
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.