Introduction: Spinal cord injury (SCI) results from a primary injury due to contusive, compressive or stretch insults followed by a multifactorial secondary one that worsen the clinical outcome[1],[2]. Among the physiopathological mechanisms involved in the progression of the lesion, inflammation is one of the most relevant[1],[2]. It is well known that microglia/macrophages can assume phagocytic activity after traumatic stimuli, and this property make polymer nanoparticles (NPs) an excellent tool for drug targeting[3],[4]. This represents an attractive strategy to exploit macrophagic cells using NPs as Trojan horses for targeted drug delivery aiming to improve drug efficacy and reduce potential side effects associated with systemic treatments.
Materials and Methods: Biodegradable NPs were obtained through a two-step process, both solvent-free, and possess a peculiar comb-like structure, where a poly-(2-hydroxyethyl methacrylate) backbone is grafted with poly-e-caprolactone and polyethylene glycol chains both controllable in terms of length and composition[5]. These NPs were tested in terms of biocompatibility, degradability and possibility to sustain the release of drugs in situ. Uptake kinetic was studied in primary cocultures of microglia, astrocytes and neurons where inflammation stimulus was induced with lipopolysaccharide (LPS)[3],[4]. Then, inflammation depletion was evaluated in vitro and in vivo using minocycline loaded NPs treatment.
Results and Discussion: We demonstrated by in vitro (Figure 1) and in vivo (Figure 2) paradigms that polymer-based NPs are very promising for the treatment of this specific cell population because they are internalized mostly by the activated form of microglia/macrophages. In particular, we proved the following: (I) NPs are captured exclusively by microglial cells in a short time (24-48 h). (II) Activated microglia (ameboid or phagocytic phenotype) are able to capture markedly these NPs, whereas resting/unstimulated cells do not. (III) NPs show a complete biocompatibility when internalized in activated microglia without inducing further proinflammatory stimuli or any toxicity. (IV) The loading of NPs with minocycline is able to reduce, in vitro (Fig. 1) and in vivo, the activation and the proliferation of microglia/macrophages around the lesion site, turning them from a round shape phagocytic-like phenotype with high CD68 level to a more arborized resting phenotype with low CD68 staining[3].

Figure. 1. NPs-Mino based treatment of activated microglia is able to revert activation process in vitro. Primary GFP-microglia cultures from mouse embryos (a), LPS activated cells (b) were incubated with Mino (c), Mino loaded in NPs (d)
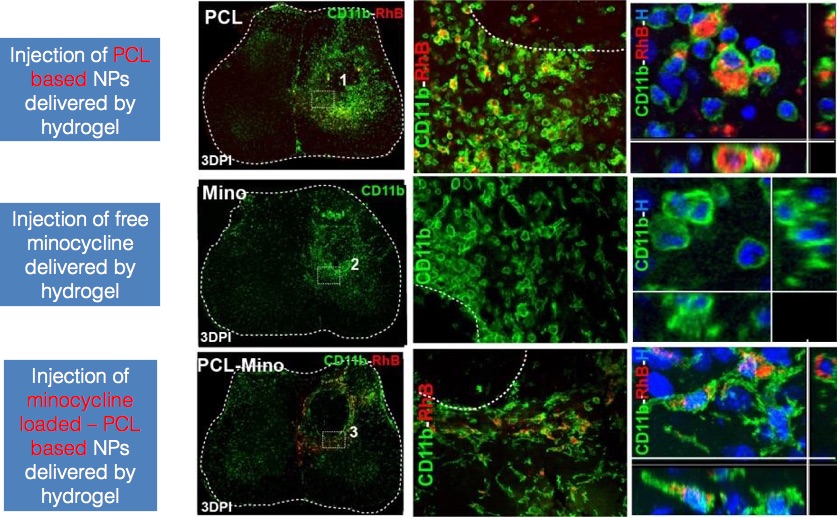
Figure. 2. NPs-Mino based treatment of activated microglia is able to revert activation process in vivo.
Conclusions: In this study we demonstrated the selective efficacy of minocycline loaded in NPs in reducing the inflammatory response mediated by microglia/macrophage activation in SCI.
References:
[1] Rossi F, Perale G et al. Expert Opinion on Drug Delivery. 10, 385, 2013.
[2] Thuret S, Moon L D F et al. Nature Reviews Neuroscience. 7, 628, 2006.
[3] Papa S, Rossi F et al. ACS Nano. 7, 9881, 2013.
[4] Papa S, Ferrari R et al. Journal of Controlled Drug Release. 174, 15, 2014.
[5] Ferrari R, Yu Y et al. Macromolecules. 44, 9205, 2011