Introduction: Glioblastoma (GBM) is the most common and lethal primary brain tumor. GBM malignancy is driven in part by self-renewing glioma stem-like cells (GSCs). GSCs are rapidly depleted in serum supplemented adherent cultures, which makes in vitro study difficult. Neurosphere culture can maintains GSCs, but sphere aggregation causes uneven access to growth factors and poor expansion of clonogenic cells[1]. We hypothesized that culturing cells in conditions that decrease sphere aggregation would drive enrichment of GSCs. To test this hypothesis, we evaluated GSC expansion in various microenvironments, including poly(N-isopropylacrylamide-Jeffamine®) (PNJ) hydrogels that we previously developed for serial passage of cells in 3D culture[2].
Methods: Patient derived GBM cells (PDCs) were obtained from patients who provided written informed consent in accordance with a protocol approved by Barrow Neurological Institute’s institutional review board. PDCs were cultured as neurospheres in suspension (control), in media solubilized hyaluronic acid (HA), polyethylene glycol (PEG), alginic acid (AA), or in PNJ hydrogels. Sphere areas were analyzed by microscopy and quantified in ImageJ. Cells were assessed for expression of GSC markers CD133 (fluorescence-activated cell sorting), Olig2, and nestin (Western blot), as well as self-renewal capacity by limiting dilution. Differentiation was induced by culture in FBS supplemented media for 2 weeks and measured by immunostaining for markers for neurons (Tubulin βIII), oligodendrocytes (GalC), or astrocytes (GFAP). Lastly, immortalized GL261-Luc cells were serially passaged in adherent, neurosphere, or PNJ scaffold conditions, evaluated for CD133 expression, and transplanted into the striatum of C57bl/6 mice. Tumor growth was evaluated by bioluminescence and hemotoxylin and eosin staining.
Results: Control cultures formed neurospheres with high variance in size that was significantly greater than PEG, AA, and PNJ cultures at 4 and 7 days (Fig 1A; whiskers = 5-95%, + = mean, significant at p<0.05, 1-way ANOVA with Tukey post-test).
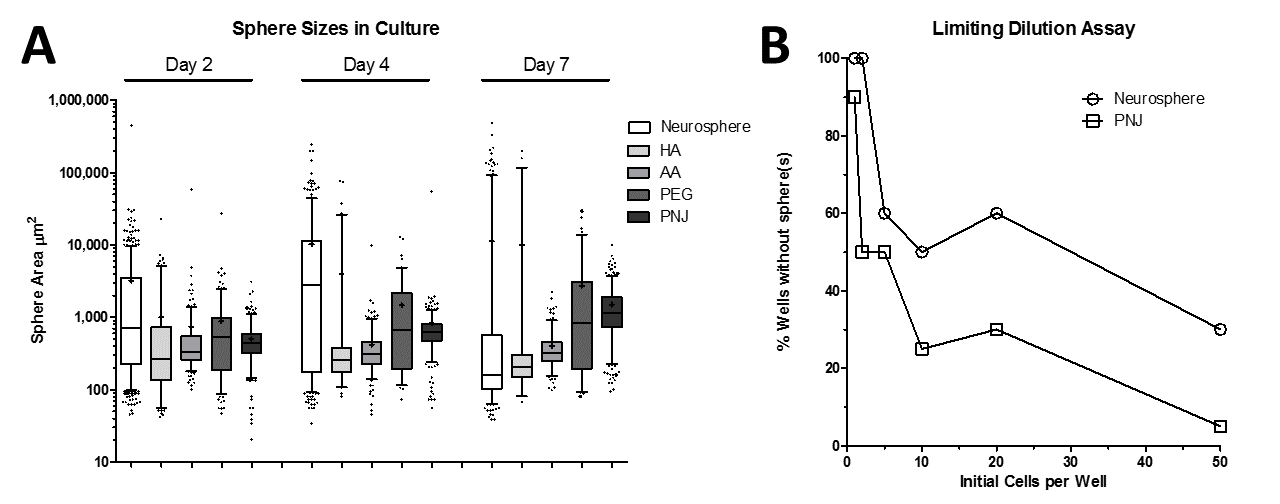
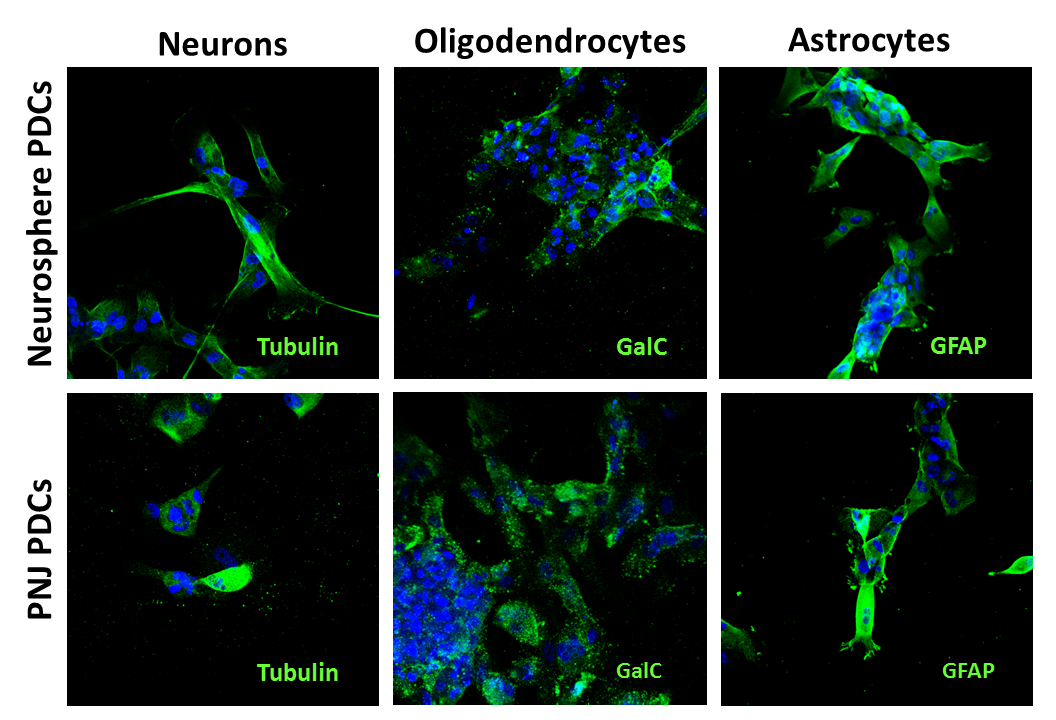
All PDC cultures maintained expression of Olig2 and nestin, but PNJ and control cultures showed improved maintenance of nonadherent neurosphere growth. CD133 expression was increased by 29% in PNJ versus control, and PNJ PDCs also exhibited a higher capacity for self-renewal (Fig 1B), which suggests specific enrichment of the GSC population. Additionally, PNJ and control cultures were both capable of differentiating into the three neuronal progenitor subtypes (Fig 2).
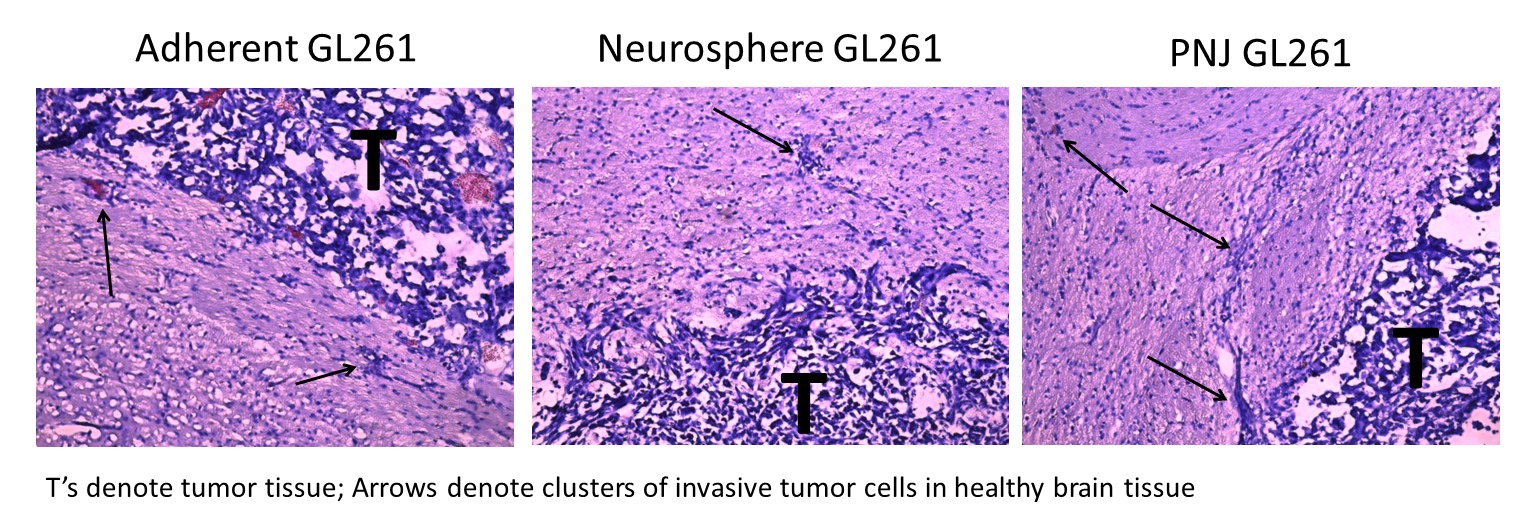
When immortalized GL261-Luc cells were serially passaged in PNJ, CD133 expression was increased 12.2 fold compared to neurosphere cultured controls. Upon transplantation into the striatum, GL261 tumors exhibit greater invasion and secondary tumor formation for PNJ cultures compared to adherent and neurosphere cultures (Fig 3). These data confirm PNJ GSC enrichment in vivo, as GSCs are more invasive than differentiated GBM cells[3].
Conclusions: Our data demonstrate enrichment of GSCs in PNJ scaffolds, which may be in part due to improved uniformity of neurosphere formation in scaffold conditions compared to controls. This approach has potential to improve GSC yield from patient samples and, perhaps more importantly, presents future opportunities for studying tumor microenvironment mediated drivers of GSC malignancy.
The authors acknowledge funding from the Barrow Neurological Foundation
References:
[1] J.M. Heffernan, D.J. Overstreet, S. Srinivasan, L.D. Le, B.L. Vernon, and R.W. Sirianni, "Temperature responsive hydrogels enable transient three‐dimensional tumor cultures via rapid cell recovery," Journal of Biomedical Materials Research Part A. 2015
[2] S.M. Pollard, K. Yoshikawa, I.D. Clarke, D. Danovi, S. Stricker, R. Russell, J. Bayani, R. Head, M. Lee, M. Bernstein, J.A. Squire, A. Smith, and P. Dirks, "Glioma Stem Cell Lines Expanded in Adherent Culture Have Tumor-Specific Phenotypes and Are Suitable for Chemical and Genetic Screens." Cell Stem Cell. 2009
[3] L. Cheng, Q. Wu, O.A. Guryanova, Z. Huang, Q. Huang, J.N. Rich, S. Bao "Elevated invasive potential of glioblastoma stem cells," Biochemical and Biophysical Research Communications. 2011