Introduction: Third generation biomaterials have been described as multifunctional as they can modulate cell behaviour and biological responses. They can contain biological materials such as cells and growth factors. Our approach aims to design a 3rd generation biomaterial for bone tissue engineering, for bone lesion filling, containing human Adipose-tissue derived Stem Cells (hASCs) and able to release bone morphogenetic protein 2 (BMP-2) in a controlled fashion. BMP-2 is one of the most common growth factors used for bone regeneration approaches. Generally, it is combined with collagen sponges, soaked in the growth factor solution before implantation. Although clinically established, it presents poor control over growth factor release. Indeed, BMPs are rapidly released to the wound site at supra-physiological concentrations, that presents concern over their clinical use[1]. Our approach consists on the development of a composite material composed of a Glycosyl-Nucleoside-Fluorinated (GNF) hydrogel core, loaded with BMP-2 and able to modulate its release kinetics, and a shell composed of collagen hydrogel containing hASCs, able to differentiate towards the osteoblastic lineage. In this work we are investigated: 1) the kinetics of BMP2 released by the core hydrogel; 2) cell survival and differentiation within the collagen shell; 3) the feasibility of the core/shell structuration in microfibers.
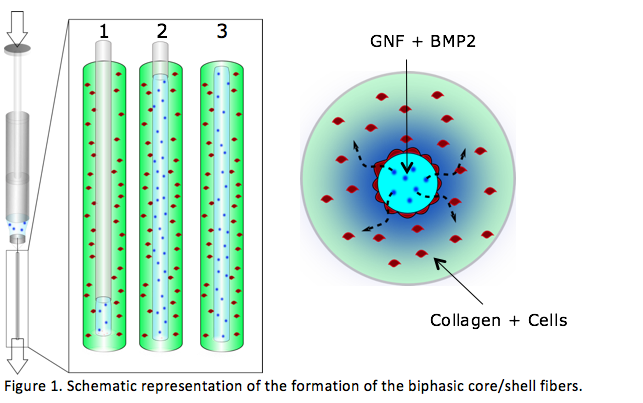
Materials and Methods: hASCs were isolated from human adipose tissue and cultured in standard conditions. GNF hydrogel was prepared as previously described[2], at a concentration of 3% (w/v). Type I-collagen was extracted from rat-tail tendons and solubilized at a concentration of 4 g/L[3]. BMP2 release was determined by an ELISA assay. hASCs survival was assessed using the live/dead assay. ALP staining was performed as means to evaluate osteogenic differentiation. To produce the composite microfibers a solution containing collagen and hASCs was injected inside a glass tube (7 mm diameter, Fig1). After collagen jellification, a syringe containing a mixture of GNF and BMP-2 was connected to the microfiber and injected by simultaneously pushing and withdrawing the syringe (Fig1, 1-3). Finally, we obtained a core of GNF/BMP2 wrapped by a shell of collagen/cells.
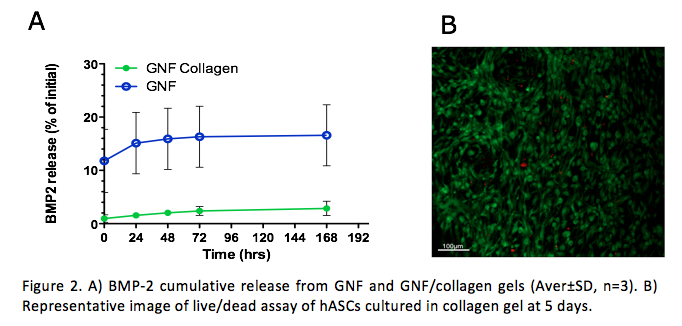
Results: To determine the impact of GNF composition over BMP-2 release we performed release studies using GNF alone or GNF/collagen blend. As observed in Figure 2A the release kinetics on GNF alone was 8 fold higher than of GNF/collagen blend, at the same time points. Nonetheless, both gels showed a sustained release up to seven days.
hASCs contained within the shell hydrogel showed to adhere and to survive up to 5 days in culture (Fig.2.B).
Discussion: Following these results, we envisage the design of a dual hydrogel-based microfiber scaffold in which the GNF core gel will release BMP-2, in a controlled fashion, to the collagen outer shell that harbours hASCs, enabling the induction of osteogenesis.
Our approach aims to merge both the use of the controlled release of growth factors and stem cells, synergistically, towards bone tissue regeneration.
This work was partially supported by NSERC-Canada, FRQ-NT-Quebec, FRQ-S-Quebec, and CFI-Canada. Mathieu Maisani was awarded of a NSERC CREATE Program in Regenerative Medicine, www.ncprm.ulaval.ca. This work was also supported by CHU de Québec Division of Regenerative Medicine
References:
[1] E. J. Carragee, E. L. Hurwitz, and B. K. Weiner, “A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned.,” Spine J., vol. 11, no. 6, pp. 471–91, Jun. 2011.
[2] S. Ziane, S. Schlaubitz, S. Miraux, A. it Patwa, C. Lalande, I. Bilem, S. Lepreux, B. Rousseau, J.-F. Le Meins, L. Latxague, P. Barthelimy, and O. Chassande, “A Thermosensitive Low Molecular Weight Hydrogel As Scaffold,” Eur. cells Mater., vol. 23, pp. 147–160, 2012.
[3] N. Rajan, J. Habermehl, M.-F. Coté, C. J. Doillon, and D. Mantovani, “Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications.,” Nat. Protoc., vol. 1, no. 6, pp. 2753–8, Jan. 2006.