Introduction: The use of hydrogels for bone tissue engineering applications can reduce the cost of patient treatment and healing time and decrease pain and discomfort of patients[1]. Hydrogels are in liquid form at the time of injection and set in situ at the body temperature and form the required shape without the need of surgery. Chitosan is a natural and biodegradable biopolymer that has been extensively studied as an organic phase of bone substitutes for bone-tissue engineering applications. Chitin or chitosan has three polymer structures; α-, β- and ⋋-chitin[2]. The α-chitin has a rigid structure made of antiparallel polymeric sheets, while the β-chitin has lower packing crystal structure weaker hydrogen bonds and parallel polymeric sheets configuration. These polymeric structural differences directly influence the biological, functional and physiochemical properties of chitosan[3]-[6]. Most of the published studies on the application of chitin in bone-tissue engineering were based on commercially available chitosan derived from crab or shrimp, and no studies have investigated the potential application of β chitosan. The aim of this study is to use chitosan extracted from squid pen and to incorporate it with different quantities of hydroxyapatite for the development of injectable gels. The biocompatibility, in vitro degradation, mechanical properties, surface morphology chemical compound and crystalline structure of the produced gels have been characterised.
Materials and Methods: Gels were made from glycerol phosphate which was mixed with crab (C) or squid pen (S) chitosan (2% w/v) and calcium phosphate compounds (CaP, hydroxyapatite and β-triclacium phosphate, HA/β-TCP) with four different concentrations (0%, 30%, 50% and 70%).
The chemical compositions and crystallinity of the produced gels have been characterised by FTIR and XRD. The surface morphology and microstructure of the gels were characterised using SEM. The physical properties (water uptake, wash out resistant and syringeability), compressive modulus and biocompatibility properties (cell cytotoxicity) of the gels have also been investigated.
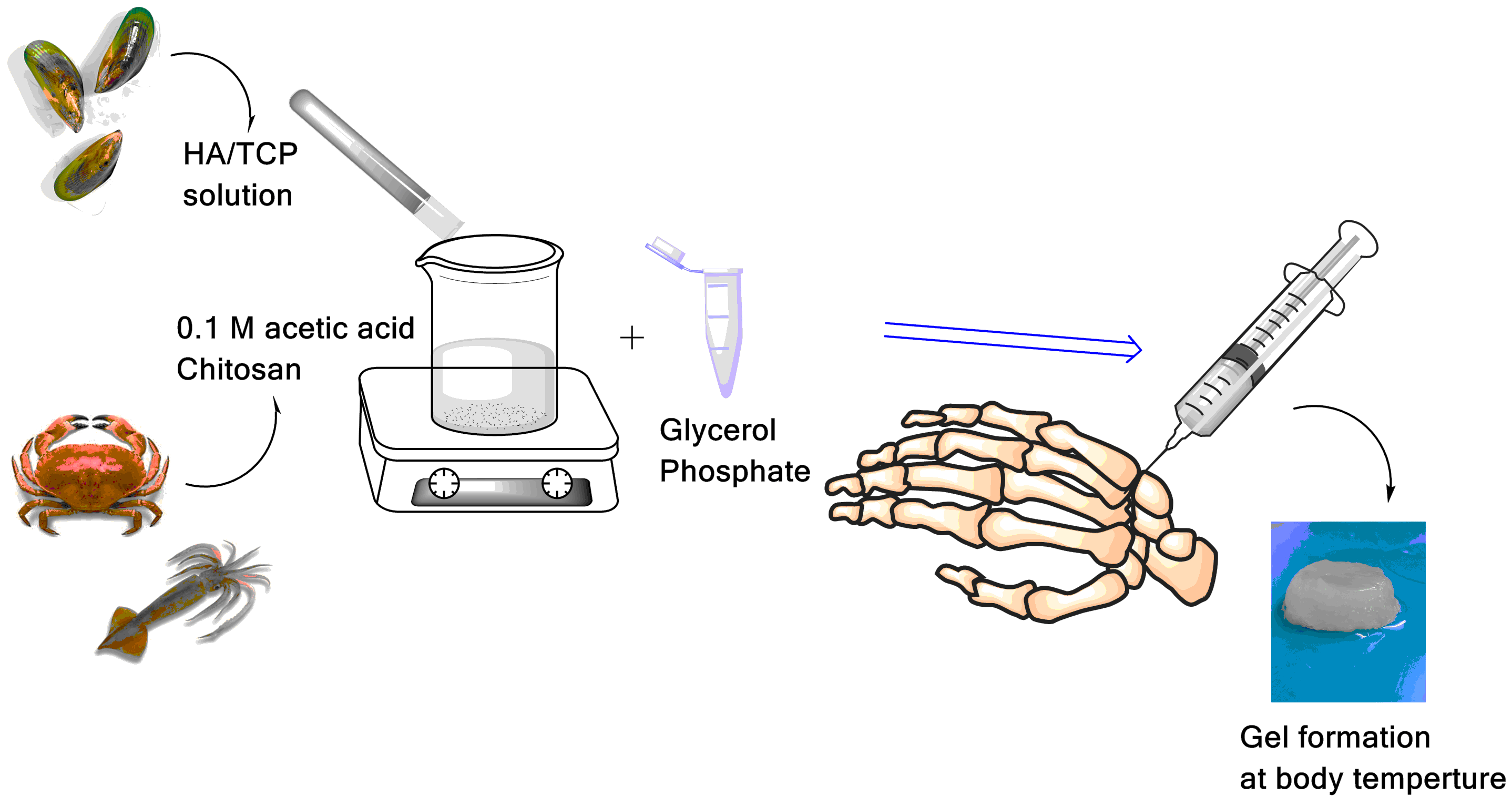
Results and Discussion: Our experimental results show that the gels rapidly settled (<3 min) and formed stable shapes at body temperature (i.e. 37°C). The S chitosan gels showed a ability of the highest water uptake (>2000%), a higher compressive modulus (up to 26 kPa) and a better cell (Saos-2) compatibility, compared to the C chitosan. More than 60% of the gel samples remained intact after contact with simulated body fluid. The C gel samples decayed faster than the S samples. For both the C and S gel samples, the number of cells on the samples with 70% of CaP was significantly (p<0.05) higher than those found in the samples with 0, 30 and 50% of CaP.
Conclusion: This study showed that S chitosan is a promising alternative to the commercially available crab/shrimp chitosan for producing injectable gels for tissue engineering applications. Squid pen chitosan gels showed higher swelling and rapider gelation compared to the C chitosan gels. Both gel samples were injectable with a required force less than 30 N. Although both gel samples showed their cytocompatibility, S chitosan gels decayed slower and showed better mechanical properties.
The authors acknowledge the facilities as well as scientific and technical assistance from staff at the Otago Centre for Electron Microscopy (OCEM) at University of Otago. We would also like to thank Mr Damian Wallas for his help and support to use XRD. The first author acknowledges the PhD scholarship awarded by University of Otago, New Zealand.
References:
[1] Huang, Z., J. Tian, B. Yu, Y. Xu and Q. Feng (2009). "A bone-like nano-hydroxyapatite/collagen loaded injectable scaffold." Biomedical Materials 4(5): 055005.
[2] Jiang, C. J. and M. Q. Xu (2006). "Kinetics of Heterogeneous Deacetylation of β-Chitin." Chemical Engineering & Technology 29(4): 511-516.
[3] Kurita, K., K. Tomita, T. Tada, S. Ishii, S.-I. Nishimura and K. Shimoda (1993). "Squid chitin as a potential alternative chitin source: Deacetylation behavior and characteristic properties." Journal of Polymer Science Part A: Polymer Chemistry 31(2): 485-491.
[4] Kumirska, J., M. Czerwicka, Z. Kaczyński, A. Bychowska, K. Brzozowski, J. Thöming and P. Stepnowski (2010). "Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan." Marine Drugs 8(5): 1567-1636.
[5] Minke, R. and J. Blackwell (1978). "The structure of α-chitin." Journal of Molecular Biology 120(2): 167-181.
[6] Dweltz, N. E. (1961). "The structure of β-chitin." Biochimica et Biophysica Acta 51(2): 283-294.