Synthesis of well-defined poly (2-oxazoline) derivatives for biomedical applications
-
1
Queen Mary, University of London, Institute of Bioengineering and School of Engineering and Materials Science, United Kingdom
Poly(ethylene glycol) (PEG) is the gold standard polymer to impart surfaces with nonfouling properties. Although PEG polymers with controlled end groups, including telechelic derivatives, is well established, their routine synthesis in the lab is challenging and hazardous. The substitution of ethylene oxide monomers with functionalized monomers with reactive side chains is also non-trivial. In addition, PEG has been reported to display immunogenic responses in vivo. Hence alternatives to PEG have attracted attention from synthetic polymer chemists. Polyoxazolines are easier to synthesise with routine laboratory equipment and can be conveniently functionalised with side chains as well end chains relatively easily[1].
Protein resistant coatings are promising in industrial and medical applications. It was reported that poly(2-methyl-2-oxazoline) shows equally excellent anti-fouling properties as PEG while poly(2-methyl-2-oxazoline) coatings are more stable towards oxidative environments[2]. Thus, Polyoxazoline is a promising alternative to PEG. This project will focus on the design of such materials with controlled end-chains, using different initiators as well as termination reagent, with well-defined poly(2-oxazoline)s architecture, to use as protein resistant polymer coatings.
In this project microwave assisted synthesis has been used for the preparation of polyoxazolines in mild conditions and characterised by NMR and FTIR. In particular the role of initiation and termination on the control of the polymer structure was investigated. Two strategies were then used for the design of polymer coatings: a grafting from approach based on methacrylate terminated poly(oxazolines) and a grafting to approach based on thiol-ene coupling of alkene functionalised polymers. The quality of polyoxazoline coatings was tested via ellipsometry and SPR (surface plasmon resonance). Moreover, fluorescence microscopy was used to chracterise protein resistance.
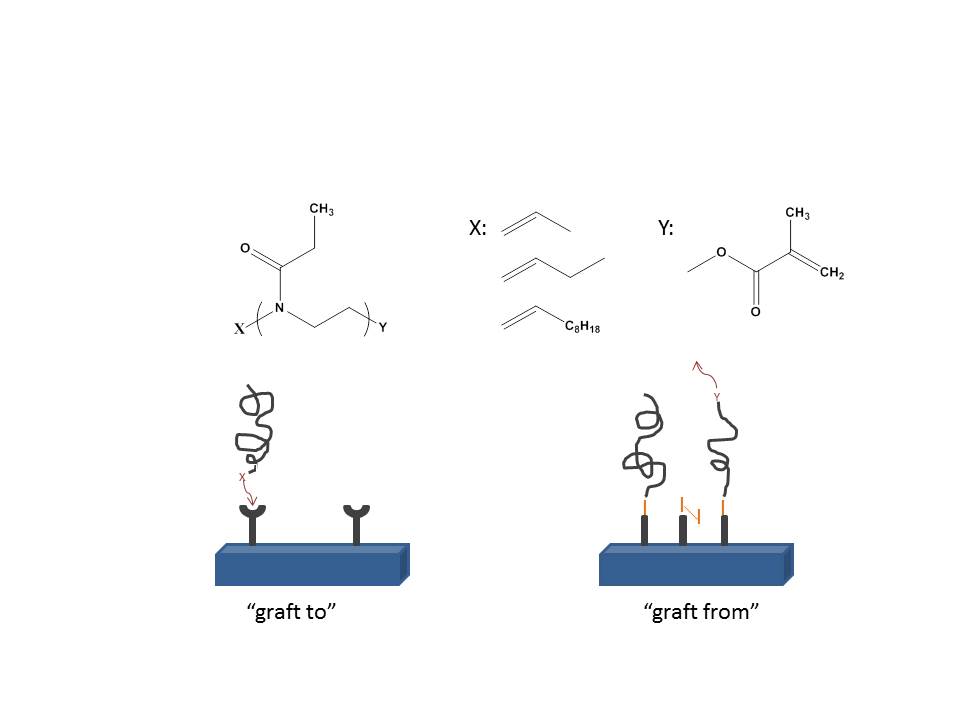
Our results show that, for low molecular weight oligomers different initiators and termination reagents can be used to control polymer architecture (NMR and MALDI-TOF). However, termination was found to be critically controlled by the temperature of the capping step. We have tried “grafting from” and “grafting to” methods with different oligomers prepared. Ellipsometry showed the successful coupling from using atom transfer radical polymerisation (ATRP) and grafting to via thiol-ene coupling, although coupling via ATRP becomes uncontrolled rapidly. The protein adhesion of key proteins used for surface functionalisation in cell based assays and present in samples analysed in biosensors (serum) was characterised via SPR and fluorescence microscopy. These results show that hydrophilic polyoxazoline coatings can be used for biosensing applications and for cell culture and patterning.
References:
[1] Knop, K., et al., Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angewandte Chemie-International Edition, 2010. 49(36): p. 6288-6308.
[2] Konradi, R., C. Acikgoz, and M. Textor, Polyoxazolines for Nonfouling Surface Coatings — A Direct Comparison to the Gold Standard PEG. Macromolecular Rapid Communications, 2012. 33(19): p. 1663-1676
Keywords:
biomaterial,
biomedical application,
polymer brush,
Polymeric material
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Naturally-derived materials and biopolymers
Citation:
Tang
P,
Colak
B,
Wang
W and
Gautrot
J
(2016). Synthesis of well-defined poly (2-oxazoline) derivatives for biomedical applications.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01451
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Pei Tang, Queen Mary, University of London, Institute of Bioengineering and School of Engineering and Materials Science, Mile End Road, United Kingdom, Email1
Dr. Burcu Colak, Queen Mary, University of London, Institute of Bioengineering and School of Engineering and Materials Science, Mile End Road, United Kingdom, Email2
Dr. Wen Wang, Queen Mary, University of London, Institute of Bioengineering and School of Engineering and Materials Science, Mile End Road, United Kingdom, Email3
Dr. Julien Gautrot, Queen Mary, University of London, Institute of Bioengineering and School of Engineering and Materials Science, Mile End Road, United Kingdom, j.gautrot@qmul.ac.uk