Introduction: Nitric oxide (NO) became a desirable molecule in the surface modification of cardiovascular implants because of its multi-physiological functions, such as anti-thrombosis and anti-hyperplasia of smooth muscle cell (SMC)[1], and the release rate and persistence of NO guarantee the long-term success rate of cardiovascular implants. So in this paper, we developed a controllable and sustained NO releasing surface for cardiovascular implants based on poly-dopamine coating loaded with different concentrations of copper ion (PDA@Cu), which have been reported able to catalyze the generation of NO from NO donor in vivo (e.g. S-nitrosothiols, RSNO) in the existence of glutathione (GSH)[2].
Methods and materials: The 316L SS samples were immersed in dopamine solution (1 mg/mL in 10 mM Tris buffer, pH 8.5) mixed with CuCl2 solution of different concentration (0, 0.1, 0.2, 0.3 mg/mL respectively) at 25° C for 12h. All the samples were ultrasonically washed in distilled water. The content of Cu in the PDA@Cu coatings was detected via XPS. Their NO catalyzing abilities were detected using Griess reagent. Platelet adhesion experiment was carried out to value their hemo-compatibility.
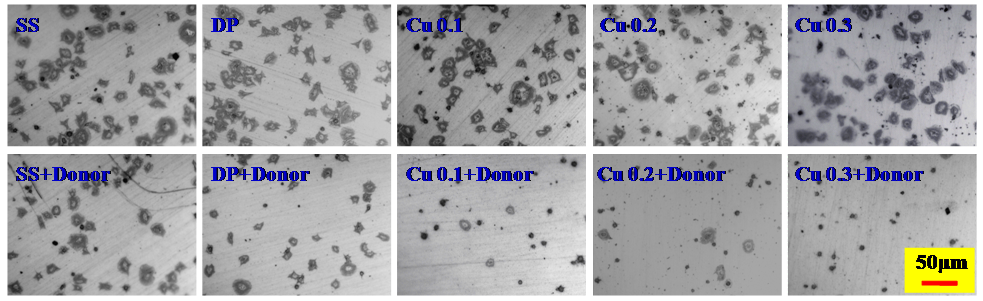
Results and Discussion: During the polymerization of dopamine, the addition of CuCl2 solution resulted in the loading of Cu ion in the PDA coatings, just as what was shown in Figure 1 A. Moreover, the Cu atomic ratios in the PDA@Cu coatings increased with the increase concentration of additive CuCl2 solution (Figure 1 C). From the high-resolution XPS spectra of Cu element (Figure 1 B), we found that when the concentration of additive CuCl2 solution increased to 0.3 mg/mL, copper ion in the PDA@Cu coatings was dominated by two valence copper, differed from the concentration of 0.1 and 0.2 mg/mL which was dominated by univalent copper. The NO catalyzing abilities of PDA@Cu coatings were shown in Figure 2. The higher Cu content, the higher NO catalyzing rate (Figure 2 A). Continued treatment of the PDA@Cu coatings with NO donor resulted in the significant decreased of NO catalyzing rate which may due to the loss of copper ion. Furthermore, the NO catalyzing abilities of PDA@Cu coatings lasted for more than 7 days (Figure 2 D). Platelet adhesion experiment was carried out to evaluate the hemocompatibility of PDA@Cu coatings, and the results were shown in Figure 3. The existence of Cu ion in PDA coating had no effect on platelet adhesion, but the catalyzed release of NO by Cu ion from NO donor resulted in significant decrease of platelet adhesion. All these results suggest that the PDA@Cu coatings have huge potential for development of cardiovascular implants.
Conclusion: During the polymerization of dopamine, the addition of CuCl2 solution resulted in the loading of Cu ion, and the content of Cu ion in PDA coatings was controllable. Cu ion in the PDA coatings had the ability to catalyze NO release from NO donor, which depended on the content of Cu ion. The catalyzing ability can last for more than 7 days.
Figure 1. Full spectrum (A), high-resolution XPS spectra of Cu element (B) and the atomic compositions and ratios of the PDA@Cu coating samples.
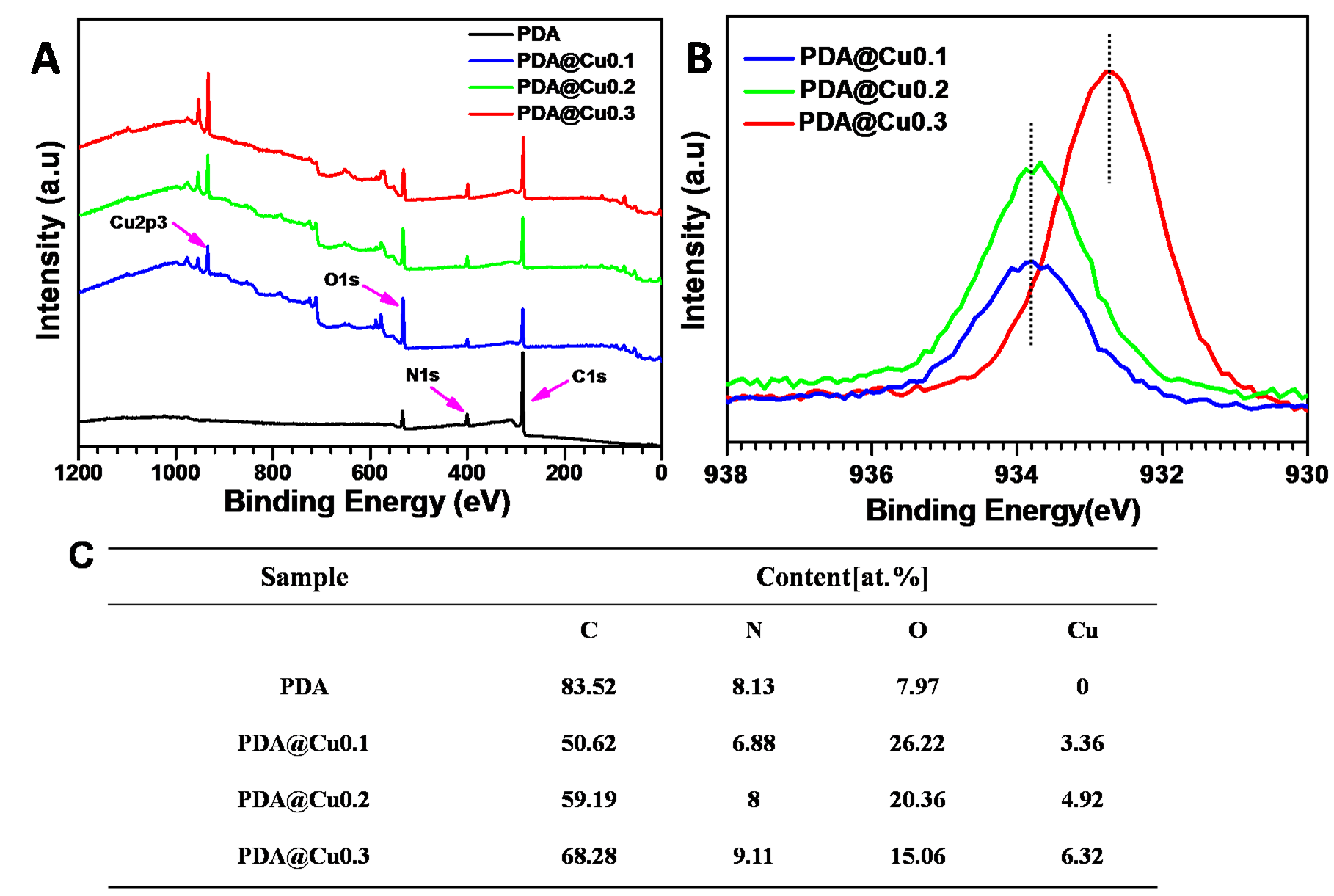
Figure 2. The NO catalyzing ability of the PDA@Cu coating samples after continued treatment with NO donor RSNO for 0h (A), 1h (B), 3d (C) and 7d (D) via Griess reagent.
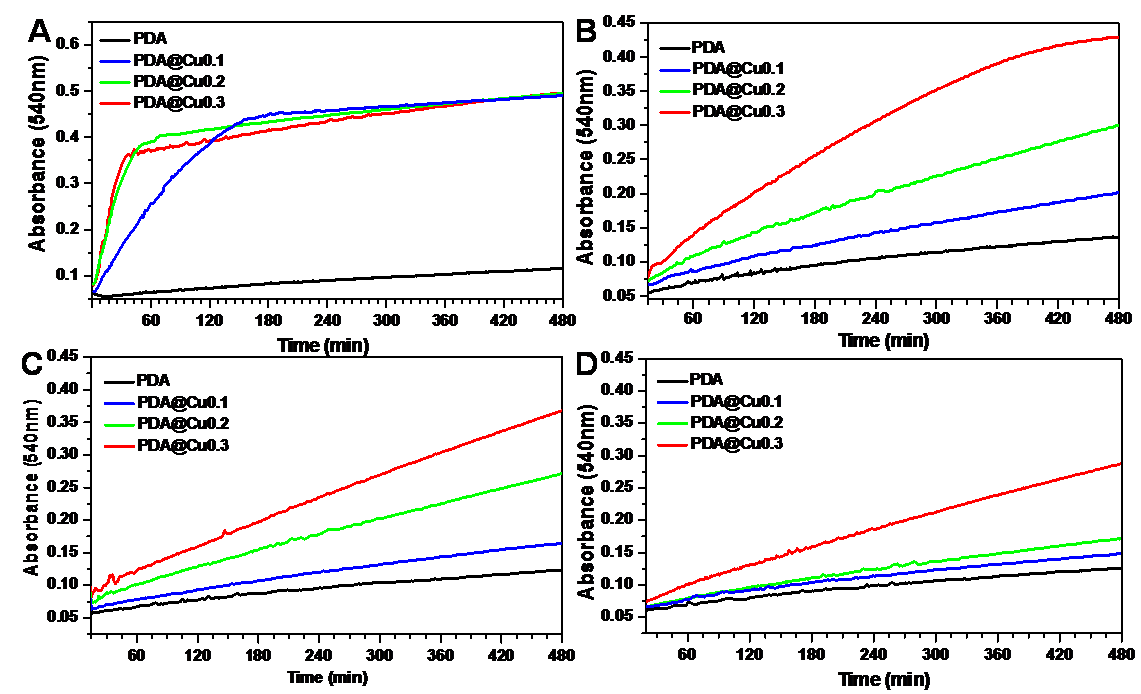
Figure 3. Platelets adhesion on 316L SS and PDA@Cu coating samples with (+Donor) and without NO donor.
This work was supported by the National Natural Science Foundation of China (Project 81271701, 31570957, 81501596 and Key Program 81330031) and the Ministry of Science and Technology of China (Key Basic Research Project No. 2011CB606204).
References:
[1] De Mel A, Murad F, and Seifalian A M. Nitric oxide: a guardian for vascular grafts? Chemical Reviews, 2011, 111(9):5742–5767.
[2] Askew S C, Barnett D J, McAninly J, Williams D L H. Catalysis by Cu 2+ of nitric oxide release from S-nitrosothiols (RSNO). J Chem Soc Perkin Trans, 1995, 2:741-745.