Introduction: In the bone marrow niche, human bone marrow stromal cells (hBMSC) are surrounded by extracellular matrix (ECM) containing CaP, collagen I, fibronectin (FN) and sulfated glycosaminoglycans (sGAG). Synthetically sulfated hyaluronan (sHA1) has been shown to support osteogenesis of hBMSC[1] and to inhibit osteoclast formation[2].
Osteoblasts synthesize large amounts of FN and deposit the protein into the bone matrix. FN is secreted as a 440 kDa dimeric glycoprotein with binding domains for cells via integrins and several ECM components like collagen I and heparin. The assembly of FN fibrils is a cell-mediated process and depends on integrin binding[3].
In this study we describe the effects of sHA1 on FN matrix assembly by hBMSC in vitro.
Materials and Methods: The sulfation of native HA was performed with SO3/DMF and resulted in sHA1with a sulfation degree of 1.3 and a molecular weight of 26,600 g/mol[4]. Fluorescence-labelled HA and sHA1 were obtained with Atto565-NH2 using side-on modification[2].
Human BMSC were cultured in DMEM with 10% FCS supplemented with 200 µg HA or sHA1 / ml[1]. For characterization of FN matrix at day 8, immunofluorescence staining was performed[1]. The influence of HA and sHA1 on FN matrix assembly was characterized using FRET-assay[5].
Results and Discussion: Immunofluorescence staining indicated a colocalization of Atto565-sHA1 and FN fibrils (figure 1C) but no colocalization of Atto565-HA and FN (figure 1B). In silico studies supported the electrostatic interaction between sHA1 and heparin binding sites which might affect growth factor binding and FN fibril formation.
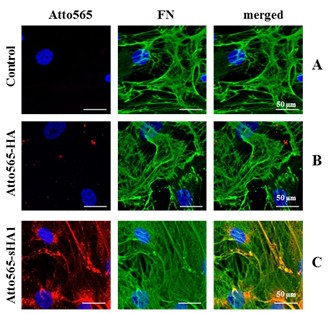
Figure 1: Immunofluorescence staining of hBMSC at day 8 (A) and after permanent treatment with Atto565-HA (B) or Atto565-sHA1 (C). FN (green), Nuclei (blue).
It was found that sHA1 affected FN matrix assembly as more and thinner FN fibrils were formed in comparison to untreated control and HA (figure 1). The effect of sHA1 on FN matrix assembly was more detailed characterized by FRET-analysis which confirmed FN-stretching in the presence of sHA1 similar to heparin[5].
Besides its effects on FN assembly, sHA1 induced FN protein and influenced its remodeling by reducing activity of matrix metalloproteinases and increasing the formation of tissue inhibitor of metalloproteinase-3 (TIMP-3)[6],[7].
Conclusion: The present study shows one putative mechanism how sHA1 influences ECM assembly and remodelling. Our findings contribute to clarify the impact of sHA1 on molecular mechanisms in hBMSC.
Deutsche Forschungsgemeinschaft TRR67 (projects B1, A7, Z3)
References:
[1] Hempel et al., Acta Biomaterialia 8: 4064-4072, 2012.
[2] Salbach-Hirsch et al., J. Cell. Biochem. 115: 1101-1111, 2014.
[3] Schwarzbauer and De Simone, Col Spring Harb Perspect Biol, 2011.
[4] Becher et al., Carbohydrate Polymers 90: 608-615, 2012.
[5] Li et al., Biomaterial Science 3: 73-84, 2014.
[6] Kliemt et al., J. Proteome Research 12: 378-389, 2013.
[7] Schmidt et al., Mol Cell Proteomics 2015, accepted.