Introduction: Using bone marrow-derived mesenchymal stem cells (MSCs) as the osteogenic cell source assures promising biological performance for bone regeneration. Hydrogel microspheres are considered attractive candidates for stem cell delivery but the current approaches to deliver MSCs are less than ideal. The project aim was to develop a new range of injectable, fast photo-curable microspheres for rapid bone repair that can provide a favorable micro-environment for MSC survival, spreading, migration and proliferation, and enhance bone regeneration.
Materials and Methods: MSCs were encapsulated in photo-crosslinkable gelatin (GelMA) microspheres using a microfluidic flow-focusing device. The resultant stem cell laden hydrogel droplets were solidified upon light exposure within a few seconds. Mechanical and degradation property of the solidified microspheres were first examined. Viability of cells encapsulated in the GelMA microspheres was measured using Live/dead® assay; spreading and migration using phalloidin/DAPI staining; proliferation using picogreen® DNA quantification assay; in vitro differentiation using alkaline phosphatase activity assay and Alizarin red. The effect of osteogenesis in vivo was investigated by injecting the cell laden microspheres into the femoral condyle of New Zealand white rabbits and the final outcome was determined using H&E and Van Gieson staining.
Results and Discussion: A microencapsulation technology generating MSC laden monodisperse GelMA microspheres with fast settability and biodegradability was developed in present study. The microspheres were found to be set within a few seconds and biodegraded by high concentration of collagenase for a week. In addition, with stem cell viability as high as 70%, the resultant GelMA microgels could support cell spreading inside the microgels and migration from the interior to the surface and enhance cell proliferation as well as regeneration (Figure 1). Moreover, the MSC-laden GelMA microspheres were found to promote osteogenesis in vitro and in vivo, associated with a significant increase in mineralization (Figure 2).
Conclusion: Injectable, photocrosslinkable, monodisperse MSC-laden hydrogel microspheres could be rapidly generated using a microfluidic flow-focusing device. The hydrogel microspheres exhibited excellent biocompatibility and osteogenic potential both in vitro and in vivo. The injectable MSC-laden GelMA microspheres could provide a micro-invasive therapy for bone regeneration.
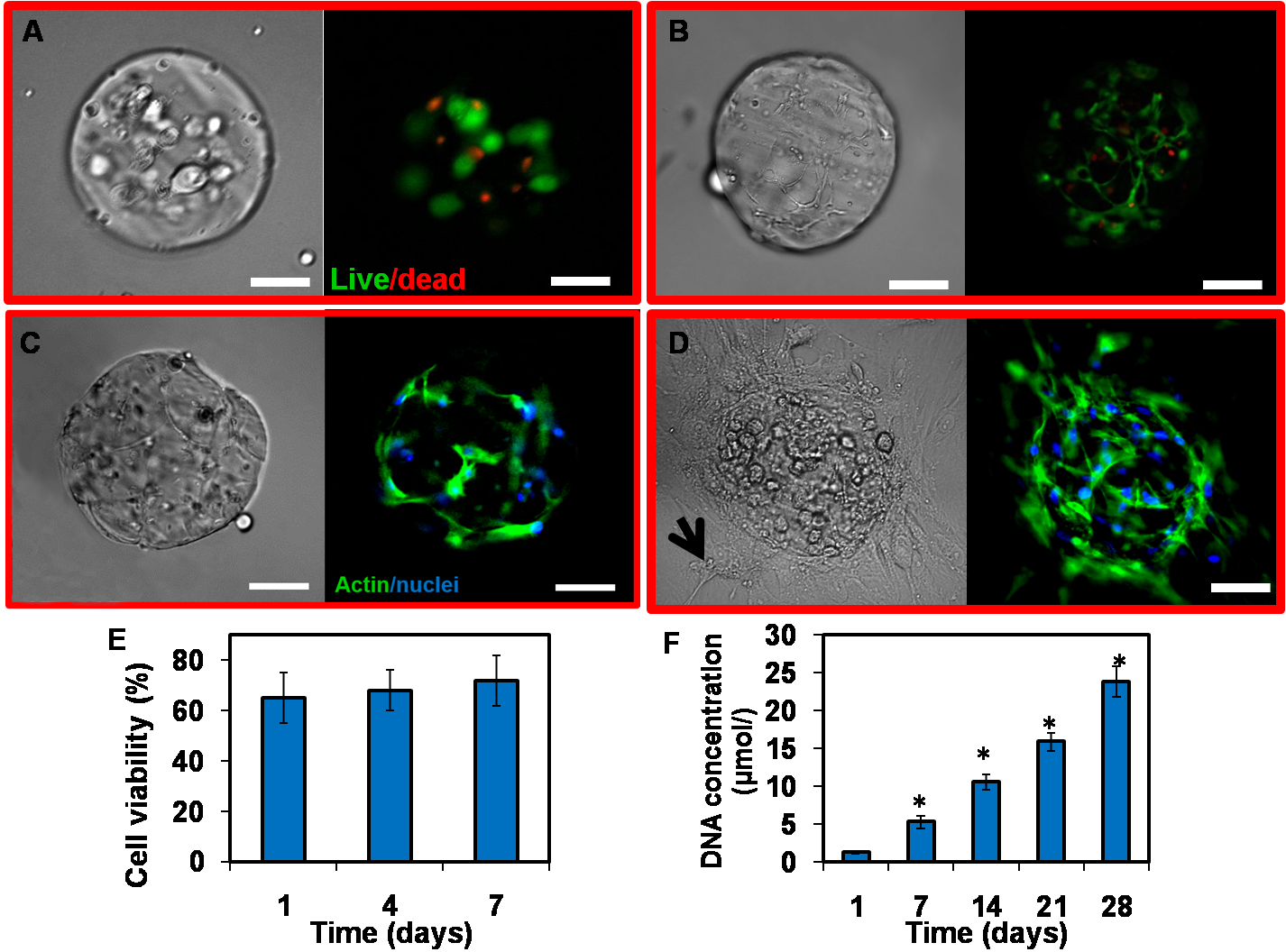
Figure 1. Viability, spreading and proliferation of hMSC encapsulated in GelMA microspheres. A and B. Live/dead® stained images of hMSC cultured in GelMA after 1 (A) and 7 (B) days. Live cells were labeled with calcein AM (green) and dead cells were labeled with ethidium homodimer. C and D. Phalloidin/DAPI images of hMSC cultured in GelMA after 2 (C) and 4 (D) weeks. Phalloidin stains cell filament green and DAPI stains cell nuclei blue. Note that cells migrate outside the microgels and attach to tissue culture plastic at 4 week (arrow). Scale bar = 100 µm. E and F. Quantification of cell viability (E) and proliferation (F).
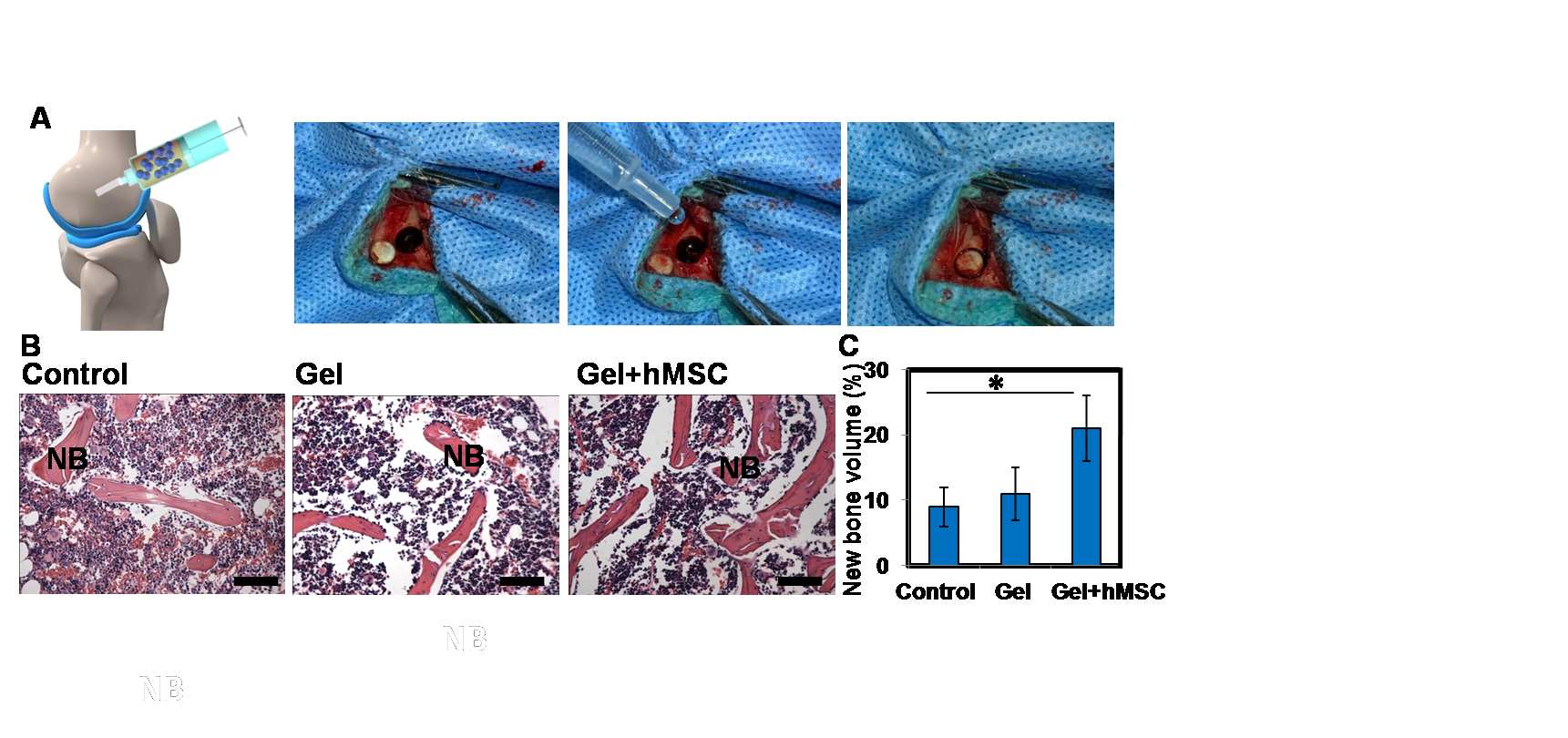
Figure 2. Bone defect repair in vivo. (A) Operation processes; (B) Histological sections of non-implanted (control), implanted GelMA microgels (gel), hMSC laden microgels (gel+hMSC) in rabbit's femur after implantation for 4 weeks stained using haematoxylin and eosin (H&E). It can be seen that the hMSC laden GelMA microgels show more new bone formation 4 weeks post surgery. “NB” indicates new bone. Scale bar = 200 µm. (C) Histomorphometrical analysis (%) of new bone formation and total area in the defect zone (*p < 0.05).
National Science Foundation