Introduction: Within the field of drug-loaded nanofibres, there has been little investigation into understanding the effect of added drug on electrospun fibre biodegradation characteristics despite evidence that added drugs have the potential to alter the rate of degradation of bulk polymer[1], microparticles[2] and even nanofibres[3],[4]. The goal of the current work was to determine the effect of adding the antibiotic ciprofloxacin (CF) and a proprietary antimicrobial polymer (AP) containing CF on the biodegradation rate of electrospun polycarbonate urethane (PCNU) nanofibre scaffolds. It was hypothesized that the added drug would affect the PCNU biodegradation rate by influencing the unique hydrogen-bonding (H-bonding) and microstructure character of the PCNU[5].
Materials and Methods: PCNU was synthesized with hexane diisocyanate:polycarbonate diol:butane diol in a molar ratio of 3:2:1[5]. The AP (7 and 15% w/w equivalent CF) or free CF-HCl (15% w/w) were incorporated via blend electrospinning. Scaffold H-bonding and microstructure were investigated via attenuated total reflectance Fourier transform IR spectroscopy (ATR-FTIR) and differential scanning calorimetry (DSC). Matrix degradation and antimicrobial release studies were carried out in PBS at 37°C for 28 days; drug release was measured by high performance liquid chromatography (HPLC), and PCNU degradation was measured using gel permeation chromatography (GPC). The cell compatibility of release products was assessed using human gingival fibroblasts (HGF), and the minimum inhibitory concentration (MIC) of released CF was measured against P. gingivalis cultures.
Results and Discussion: CF from the AP scaffold was released slowly over the duration of the study, while drug aggregation within the 15% w/w CF-HCl scaffolds led to a burst release (Fig.1). The addition of drug caused an increase in the hydrolytic stability of the PCNU scaffolds, proportional to the amount of drug added, regardless of the form it was added (Fig.2).
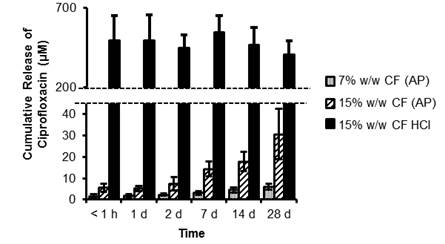
Fig.1. Cumulative release of CF in PBS (pH ~ 7) at 37°C (μM). Data are mean ± SD (n=9).
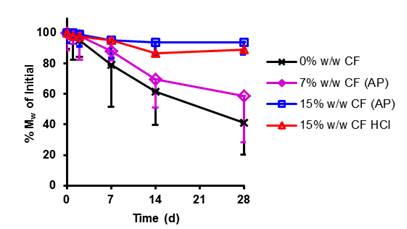
Fig.2. Reduction in polystyrene equivalent weight average molecular weight (Mw), as a percent (%) of original (t=0 days), determined by GPC. Data are the mean ± SD (n=9).
At day 28, H-bonding (FTIR) within the hard-segment and phase mixing (DSC) had decreased for the 0% w/w CF scaffolds and increased for the drug-loaded scaffolds (data not shown). Increased H-bonding and phase mixing for the latter may have led to shielding of the hydrolytically susceptible carbonate soft-segment, with the result that the drug-loaded scaffolds had greater stability. Accumulated PCNU biodegradation products (regardless of drug presence) significantly decreased HGF relative metabolic activity (Fig. 3). The MIC of released CF was higher than that of CF-HCl off-the-shelf (~0.5 µg/mL vs. 0.25 µg/mL respectively).
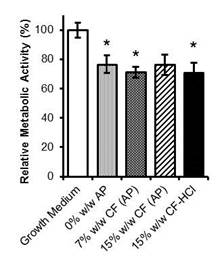
Fig.3. Relative metabolic activity as a % of the growth medium control of Day 7 scaffold release products. * Represents significant difference from growth medium control (p < 0.05). Data are the mean ± SE (n=9).
Conclusion: The results indicate the ability to use AP to generate electrospun scaffolds with a sustained (>28 days) release of CF. The combination of sustained drug release and delayed scaffold degradation presents a viable treatment modality for medical device applications requiring long term antimicrobial management (e.g. post-surgical oral wounds).
National Science and Engineering Research Council (NSERC) Synergy; NSERC CGS D; IBBME; Interface Biologics Inc. for supply of AP
References:
[1] S. J. Siegel et al., Eur. J. Pharm. Biopharm., vol. 64, no. 3, pp. 287–93, Nov. 2006.
[2] J. M. Anderson and M. S. Shive, Adv. Drug Deliv. Rev., vol. 64, pp. 72–82, Dec. 2012.
[3] W. Cui, et al., Biomacromolecules, vol. 7, pp. 1623–1629, 2006.
[4] Z.-M. Huang, et al., J. Biomed. Mater. Res. A, vol. 77, no. 1, pp. 169–79, Apr. 2006.
[5] Y. W. Tang, et al., J. Biomed. Mater. Res., vol. 56, no. 4, pp. 516–28, Sep. 2001.