Introduction: Biological apatite presented in bone and teeth of mammals contains various ions. Substitution of these ions into hydroxyapatite (Ca10(PO4)6(OH)2; HAp) induces lots of strains and defects in the crystal structure. Generally, autogenous-bone graft shows the better clinical score than artificial-bone grafting. This result suggests the possibility that the defects of apatite in autologous bone could induce osteoinduction[1]. Up to now, we have successfully synthesized HAp powders including bone minerals (hereafter, bone HAp) as models for biological apatite[2]. In this study, in order to clarify the effect of substitution of the bone minerals into HAp on the bioresorbability, we compared bioresorbability of porous bone HAp ceramics with that of stoichiometric HAp (pure HAp).
Materials and Methods: As previous reported[2], bone HAp powders were synthesized by a wet process. Pure HAp powders as control were also synthesized by the above process. The bone or pure HAp porous ceramics were fabricated by firing the compact using carbon beads as porogen at 1300 °C for 5 h under steam and CO2 gas atmosphere. The material properties were determined by an X-ray diffractometry (XRD), inductively coupled plasma-atomic emission spectroscopy (ICP-AES). In addition, the dissolution of Ca2+ ions released from the specimens were measured using Ca2+ ion electrode[3]. Bioresorbability and biocompatibility were examined by implanting the samples into tibia of pig (wild type). At 12 weeks after implantation, the samples were prepared for histological analysis. The rates of bioresorption and bone formation were calculated by imaging software.
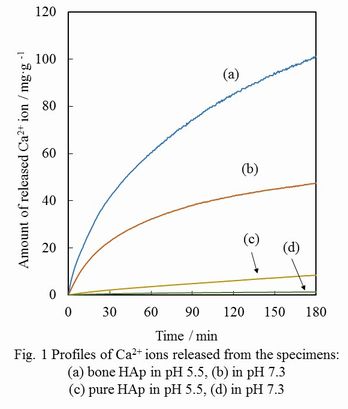
Results and Discussion: The XRD patterns of bone HAp or pure HAp porous ceramics indicated that crystalline phases were HAp single phase. Furthermore, the presence of Na+, K+ and Mg2+ ions was confirmed in bone HAp by ICP-AES. Under both acidic and neutral conditions (pH 5.5 and 7.3), the dissolution of Ca2+ ions released from bone HAp was much higher than that of pure HAp (Fig. 1). This suggests that the bioresorbability of bone HAp would be higher than that of pure HAp.
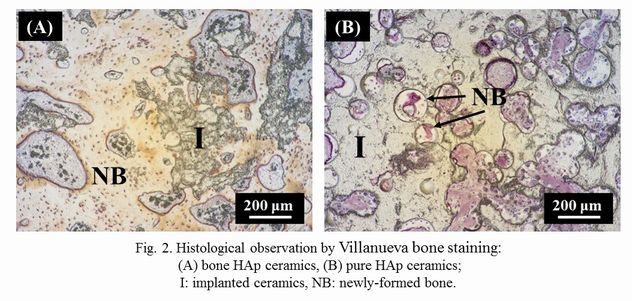
Histological observation by Villanueva bone staining showed that pure HAp remained after implantation (Fig. 2). By contrast, bone HAp were absorbed and replaced with newly-formed bone. The bioresorption rate of bone HAp was significantly more than that of pure HAp. The results in vivo bioresorption correspond to the above-mentioned ones of in vitro solubility. The bone formation rates of bone and pure HAp ceramics was 34.1 and 13.1%, respectively. This may be due to the high resorbability of bone HAp ceramics on the basis of faster bone-remodeling cycle compared to that of pure HAp ceramics.
Conclusion: In the present study, we have demonstrated that porous bone HAp ceramics have higher bioresorbability compared to porous pure HAp ceramics. Thus, this material would be promising one of the candidates as a novel bioresorbable artificial bone.
References:
[1] M. Aizawa, N. Kanzawa and M. Matsumoto, J. Jpn. Soc. Biomater., 23, 336-342 (2005).
[2] M. Aizawa, T. Miki, Y. Yasutomi, M. Honda and H. Yoshimura, Key Engineer. Mater., 493-494, 320-324 (2012).
[3] Japanese Industrial Standards. JIS T 0330-3:2012(J).