Dual use of 3D printed and micropatterned polycaprolactone scaffolds for in vivo guidance of oriented tissue formation in the bone-ligament complex
-
1
University of Michigan, Department of Biomedical Engineering, United States
-
2
University of Michigan, Department of Periodontics and Oral Medicine, United States
Introduction: Development of scaffolds with micro-scale cues for guided alignment of collagenous tissue formation that interfaces with osseous tissue is needed for bone-ligament complex regeneration. Micropatterned substrates have been assessed for cellular alignment to promote aligned collagen fiber synthesis in vitro[1]. However, there is lack of research regarding conditions necessary for patterning to promote in vivo aligned tissue formation. We use 3D-printing and micro-engineered topography to create polycaprolactone (PCL) scaffolds to present macro- and micro-level features for guidance of bulk osseous and soft tissue formation in vivo. The focus of this study is to mimic the periodontal ligament (PDL)-alveolar bone complex in the oral cavity, which is susceptible to damage via chronic inflammation from periodontitis—a leading cause of tooth loss. The design comprises of a hPDL cell-seeded PCL film patterned with 250um high, grooved pillars consistent with human PDL parameters, and a 3D-printed PCL scaffold. Effects of topography on cell alignment and collagenous tissue formation in vivo are assessed by varying pillar groove width (15um, 60um) and depth (10um, 30um).
Materials and Methods: PCL films were patterned using a customized mold fabricated via soft lithography and PCL scaffolds were 3D-printed using selective laser sintering. Scanning electron microscopy was used to visualize 3D patterns on PCL films (Fig 1a). Films were hydrolyzed and fibronectin-treated prior to cell seeding and 3D printed PCL region was seeded with adenoviral BMP7-transduced fibroblasts prior to subcutaneous implantation using an existing model[2]. Bone volume and tissue mineral density (TMD) were analyzed using micro-CT at 3 and 6 weeks post-implantation. Aligned collagen thickness was assessed using H&E and Masson’s trichrome staining; tubulin and DAPI staining was performed for cellular elongation and nuclear orientation quantification (ImageJ).
Results and Discussion: Our data indicates that percentage of aligned cells in vivo on 30um deep pillar grooves (35±8.3% to 39±6.8% for 60 and 15um wide grooves, respectively) was significantly higher compared to 10 um deep grooves (24±8.7% to 25±6.4%), including non-grooved pillars (15±7.0%) and porous PCL (9.1±3.6%) (Fig 1b). Pillars with 30um deep grooves had significantly (p<0.0001) higher oriented collagen thickness (29.1±10.0 to 33.7±15.1um) compared to non-grooved pillars (16.5±6.7um). These results indicate importance of groove depth over width as a measure of cellular alignment and collage formation in vivo. Significant (p<0.001) increases in bone TMD from weeks 3 to 6 were also observed with no changes in bone volume.
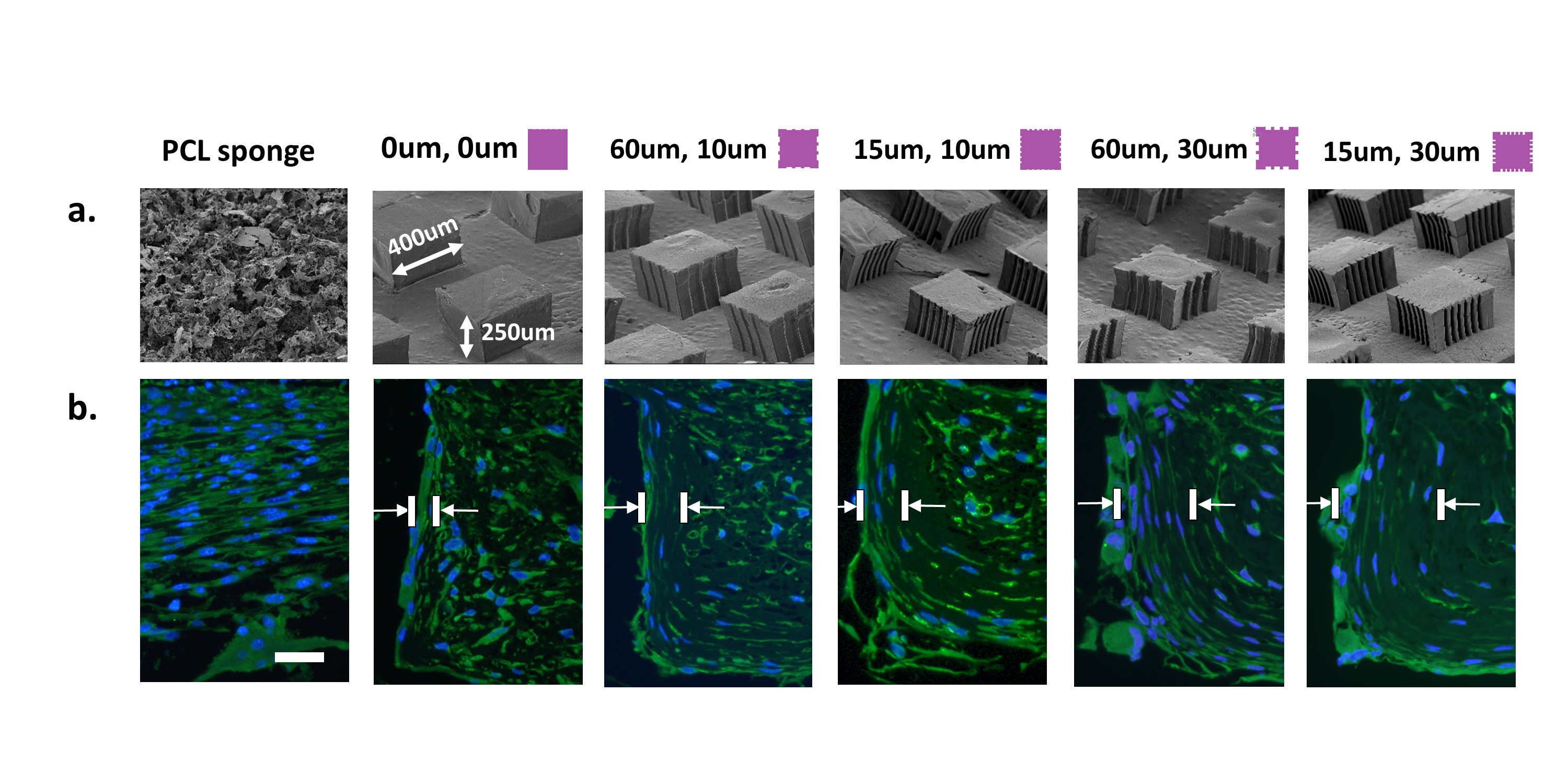
Figure 1: (a) Designed 3D topography of ligament-specific PCL scaffold region with random orientation, non-grooved pillars, and pillars with varying groove depth (10-30um) and width (15-60um); (b) DAPI, tubulin staining of tissue at 6 weeks post-implantation shows increased cellular alignment with increasing groove depth (scale bar = 50um).
Conclusion: We developed a scaffold with 3D-printed and micropatterned regions capable of supporting bone formation and oriented collagenous tissue formation in vivo. Micro-engineering topography of the ligament-specific scaffold region has direct effects on cellular alignment and elongation. Such a design-oriented approach has potential for improving the regenerative response of bone-ligament complexes in interface tissue engineering.
References:
[1] Bettinger CJ et al. Angewandte Chemie. 2009;48:5406-15.
[2] Park CH et al. Biomaterials. 2010; 31:5945-52.
Keywords:
microstructure,
material design,
3D scaffold,
complex tissue orgnization
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Synthetic scaffolds as extracellular matrices
Citation:
Pilipchuk
S,
Monje
A,
Jiao
Y,
Hao
J,
Kruger
L,
Flanagan
CL,
Hollister
SJ and
Giannobile
WV
(2016). Dual use of 3D printed and micropatterned polycaprolactone scaffolds for in vivo guidance of oriented tissue formation in the bone-ligament complex.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01159
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Alberto Monje, University of Michigan, Department of Periodontics and Oral Medicine, Ann Arbor, MI, United States, Email1
Dr. Yizu Jiao, University of Michigan, Department of Periodontics and Oral Medicine, Ann Arbor, MI, United States, Email2
Dr. Jie Hao, University of Michigan, Department of Periodontics and Oral Medicine, Ann Arbor, MI, United States, Email3
Dr. Laura Kruger, University of Michigan, Department of Periodontics and Oral Medicine, Ann Arbor, MI, United States, Email4
Dr. Scott J Hollister, University of Michigan, Department of Biomedical Engineering, Ann Arbor, MI, United States, Email5
Dr. William V Giannobile, University of Michigan, Department of Biomedical Engineering, Ann Arbor, MI, United States, Email6