Introduction: Biodegradable aliphatic polyesters such as poly(lactide) (PLA) and poly(ε-caprolactone) (PCL), largely used in tissue engineering applications, lack of suitable functional groups and biological clues to enable interactions with the cells[1],[2]. Due to the ubiquity of thiol groups in biological environment, and to the versatility of thiol chemistry[3], we addressed this issue with the design and synthesis of poly(lactid acid) chains bearing pendant thiol-protected groups. To achieve this, poly-condensation reactions of suitably prepared sulfur-functionalized hydroxy-acids were performed, affording, however, low molecular weight samples[4]. Recently, we succeed in the synthesis of a lactide-type monomer carrying a pendant thiol group, and used it in controlled ring-opening polymerization (ROP)[2] as a “building block” for the functionalized porous scaffolds, that were finally covalently linked to -RGD sequences.
Materials and Methods: Moisture and air-sensitive materials were manipulated under nitrogen using Schlenk techniques or a MBraun Labmaster glovebox. Reagents and solvents were purchased from Sigma-Aldrich. The monomer precursor[4] and the catalyst[5] have been reported in previous papers, as well as procedures for the polymerization tests[5]. The poly(ε-caprolactone-co-L-lactide) (PCLA) and the scaffolds preparations were performed as previously described[6]. The H-Arg-Gly-Asp-Cys-OH peptide (RGDC) was purchased from Bachem and used as received.The characterization methods have been also previously described[5],[6]. The viability of human dermal fibroblasts cultured with the extraction medium was determined using an Alamar blue assay.
Results and Discussion: The thiol-functionalized monomer was synthetized in 3 steps, starting from L-lactide, with a good yield. The controlled ring-opening co-polymerization of the thiol-lactide with L-lactide and ε-caprolactone, in the presence of a dimethyl(salicylaldiminato)aluminum catalyst[5], afforded the thiol-functionalized PLA and PCLA. The polymeric samples were fully characterized by NMR, SEC and DSC analysis. Exploiting a disulphide exchange reaction, pyridylthio groups were added as pendant functionalities. After blending with PCLA, porous scaffold were obtained by salt leaching method. The DSC thermogram of the scaffolds made with the blend (10 % in thiol-functionalized copolymer) showed a unique glass transition, indicating that the copolymers were miscible in a single phase.
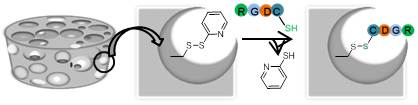
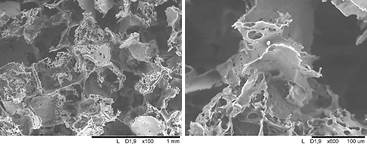
The pyridylthio groups displayed fast and selective reactivity with cysteine-terminated RGD peptide (Figure 1). SEM surface images (Figure 2) showed highly porous morphology. Moreover, the cytotoxicity test showed good cell viability.
Conclusion: The disclosed method represents a fast and efficient way toward biodegradable aliphatic polyesters with pendant editable side groups. The reaction with the RGDC is a proof of the concept, and demonstrates the potential to graft any thiolated ligands to the aliphatic poly(esters) main chain. We believe these materials have great potential to be used in several biomedical applications, where both presence of functionalities and biodegradability are required.
DP thanks for funding VINNOVA, Mobility for Growth, and the Marie Curie Actions FP7-PEOPLE-2011-COFUND (GROWTH 291795).; The Swedish Research Council (Dnr 621-2013-3764) is gratefully acknowledged.
References:
[1] R. Langer, J.P. Vacanti, Science 1993, 260, 920–926
[2] A.-C. Albertsson, I. K. Varma, Biomacromolecules 2003, 4, 1466-1486
[3] (a) C. E. Hoyle, A. B. Lowe, C. N. Bowman, Chem. Soc. Rev. 2010, 39, 1355–1387. (b) M. Le Neindre, R. Nicolay, Polym. Chem. 2014, 5, 4601-4611
[4] D. Pappalardo, S. Målberg, A. Finne-Winstrad, A.-C. Albertsson, J. Polym. Sci., part A, Polym. Chem. 2012, 50, 792-800
[5] D. Pappalardo, L. Annunziata, C.Pellecchia, Macromolecules 2009, 42, 6056–6062
[6] S. Dånmark, A. Finne-Wistrand, M. Wendel, K. Arvidson, A.-C. Albertsson, K. Mustafa, J. Bioact. Compat. Polym. 2010, 25, 207-223