Introduction: The outcome of patients with coronary artery disease has been significantly improved by percutaneous coronary interventions with stent implantation. However, despite progress made on stents and with the antithrombotic treatments, stent thrombosis remains an important issue because of its serious adverse consequences[1],[2]. Several mechanisms favor stent thrombosis such as platelet aggregation, fibrin formation, defective healing and local inflammatory responses[3]-[6]. In this context, we investigated in vitro the thrombogenicity, proinflammatory properties and healing capacities of a cobalt-chromium (CoCr) alloy.
Materials and Methods: The study was performed on bare metal (electropolished CoCr discs from Biotronik SE & Co) as the backbone of new generations of stents. Platelet adhesion under flow conditions was quantified and platelet receptors were determined using specific antibodies. Thrombin generation was performed using the Calibrated Automated Thrombogram in the presence or absence of platelets. Neutrophil adhesion and formation of extracellular traps (NETs) were visualized by scanning electron microscopy and immunofluorescence using histone H1 labelling. Endothelialization of CoCr was performed using primary Human Coronary Artery Endothelial cells (HCAEC). Confluence and viability of the cells were analyzed by phalloidin labeling and by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) test, respectively. The phenotype of the cells was analyzed using specific antibodies against PECAM-1 (CD31), ICAM-1 (CD54) and VCAM-1 (CD106) and the procoagulant potential was analyzed by thrombin generation and protein C activation.
Results and Discussion: Our results show that human blood platelets adhered to and are activated on CoCr in both static and flow conditions. CoCr also activates the contact pathway of coagulation resulting in thrombin formation that is enhanced by platelet activation. CoCr triggers leukocyte adhesion and acts as a scaffold for the formation of neutrophil extracellular traps in the presence of platelets. Primary human coronary endothelial cells adhere, proliferate and form a monolayer covering CoCr. However, they switch from an anticoagulant phenotype to a procoagulant one with a reduced capacity to trigger activation of the anticoagulant protein C and an increased expression of tissue factor, the main trigger of coagulation. Endothelial cells grown on CoCr also acquire an inflammatory phenotype as indicated by their increased expression of ICAM-1 and VCAM-1.
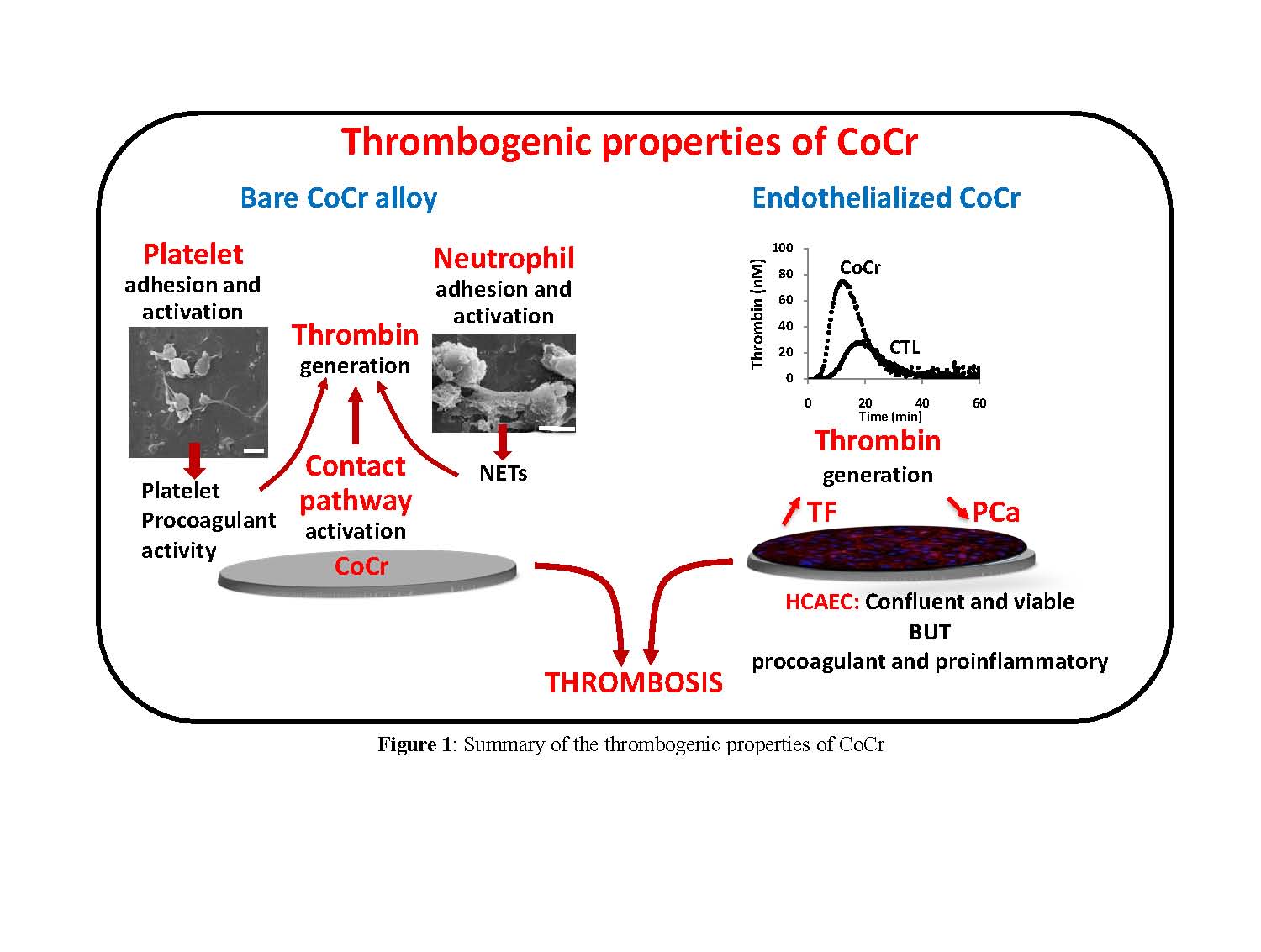
Conclusion: We demonstrate that bare CoCr is prothrombotic and proinflammatory due to its capacity to activate platelets and coagulation and to induce leukocyte adhesion and activation. Even if endothelialization is obtained, the switch in endothelial phenotype prevents effective healing. We propose our methodology for future preclinical in vitro evaluations of the thrombogenicity of stent materials.
References:
[1] Fischman, D.L., et al., A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med, 1994. 331(8): p. 496-501.
[2] Serruys, P.W., et al., A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med, 1994. 331(8): p. 489-95.
[3] Brash, J.L., et al., Mechanism of transient adsorption of fibrinogen from plasma to solid surfaces: role of the contact and fibrinolytic systems. Blood, 1988. 71(4): p. 932-9.
[4] Joner, M., et al., Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol, 2006. 48(1): p. 193-202.
[5] Fuchs, T.A., et al., Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A, 2010. 107(36): p. 15880-5.
[6] Finn, A.V., et al., Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol, 2007. 27(7): p. 1500-10.