Bioactive modification of decellularized matrix for vascular applications
-
1
Oregon Health & Science University, Biomedical Engineering, United States
-
2
Oregon Health & Science University, Knight Cardiovascular Institute, United States
-
3
Oregon Health & Science University, Department of Neuroscience, Oregon National Primate Research Center, United States
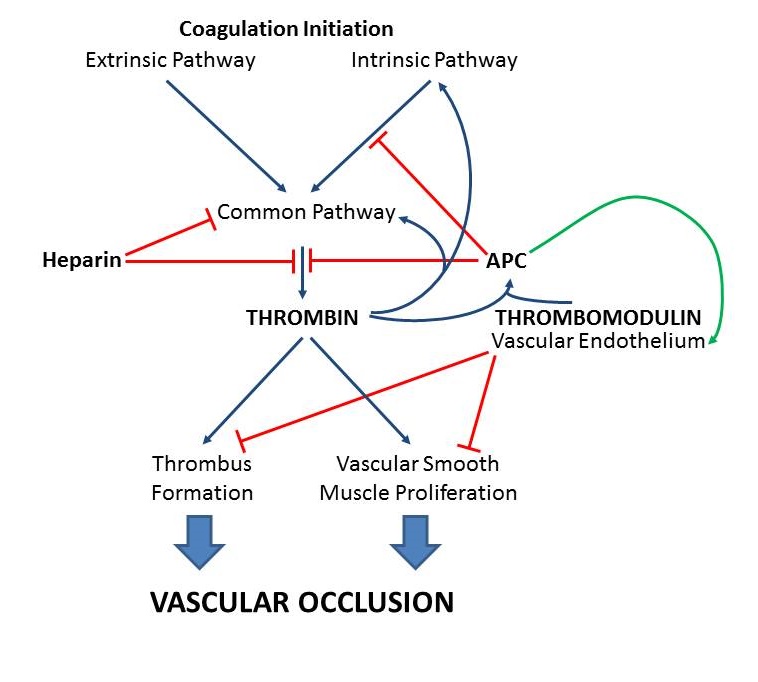
Introduction: Decellularized matrix derived from porcine small intestinal submucosa (SIS) is a widely-used biomaterial being investigated for vascular applications including vascular grafts, stent coverings and artificial venous valves. However, the success of SIS-based vascular devices has been limited in some cases due to thrombus formation as well as intimal hyperplasia resulting from unregulated proliferation of vascular smooth muscle cells. To address these issues, this work modifies SIS with two bioactive molecules: heparin and the endothelial cell protein thrombomodulin (Figure 1). Heparin markedly accelerates antithrombin-mediated inhibition of thrombin and coagulation factor X, while thrombomodulin is the critical cofactor for generating activated protein C (APC). APC directly inhibits coagulation and also promotes endothelial cell regulation of intimal hyperplasia. This work determines the ability of these modifications to enable APC generation and inhibit coagulation on SIS.
Materials and Methods: SIS (Cook Biotech) was modified via adsorption by soaking in solutions of thrombomodulin (4 µg/ml) or heparin (100 µg/ml) alone or in combination. Prior to all studies, modified SIS was washed with Tris-buffered saline. APC generation was measured by incubating modified SIS in a solution of protein C and thrombin for 1 hr at 37°C. Following inhibition of thrombin with hirudin, APC activity was quantified using a chromogenic substrate. To determine the anticoagulant activity of the modifications, modified SIS discs were soaked with either tissue factor or a contact pathway activating solution, then citrate-anticoagulated platelet poor plasma was added on top of the SIS and recalcified. The time to coagulation measured optically using a plate reader. A one-way ANOVA with Tukey's post hoc was used to determine significant differences between groups, with p < 0.05 considered significant. Data are presented as the mean ± standard deviation, letters indicate homogenous subsets based on the post hoc analysis.
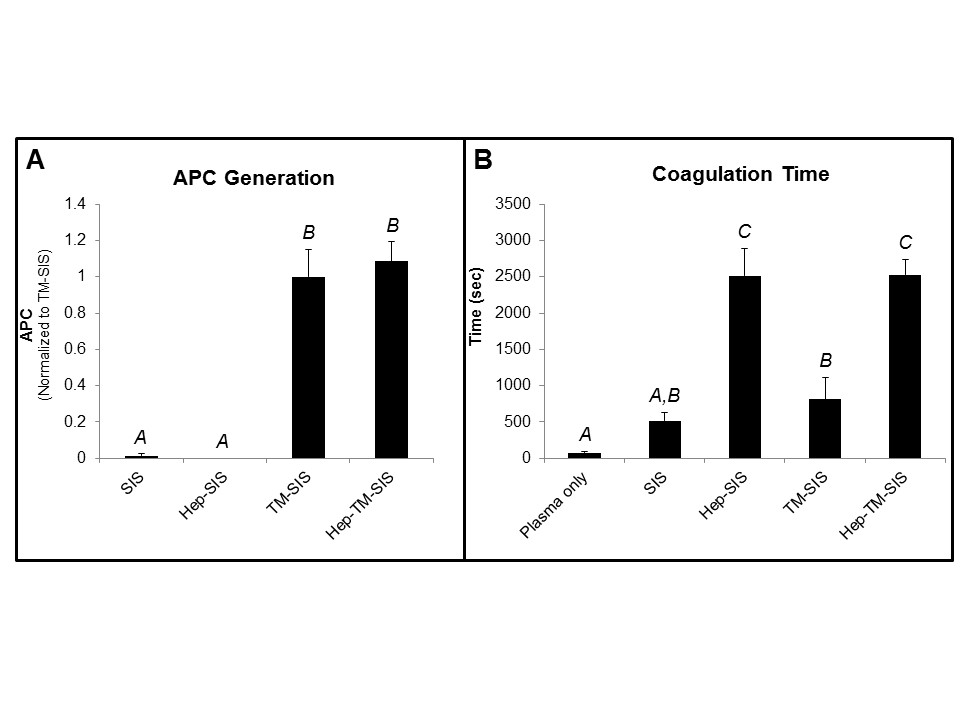
Results and Discussion: Thrombomodulin-modified SIS catalyzed APC generation at a rate of 23.49 ± 2.44 µg/cm2·hr. Modifying SIS with heparin prolonged blood plasma coagulation initiated by either tissue factor or contact pathway activation. A dual modification, where SIS is first modified with thrombomodulin then with heparin, enabled both APC generation (Figure 2A) and prolonged tissue factor-initiated blood coagulation (Figure 2B) similar to either modification done independently. These results indicate that modifying SIS with both thrombomodulin and heparin may improve the material's hemocompatibility by inhibiting blood coagulation as well as by catalyzing APC generation that may result in APC-mediated endothelial cell signaling. Ongoing studies are utilizing a baboon ex vivo arteriovenous shunt with flowing whole blood to quantify platelet accumulation and fibrin deposition on modified SIS devices.
Conclusion: This work successfully modified SIS with the bioactive molecules heparin and thrombomodulin, thereby enabling APC generation on the material as well as prolonging blood plasma coagulation. This modified SIS is a promising biomaterial for vascular applications due to its anticoagulant activity and APC generation, which may support endothelial cell inhibition of intimal hyperplasia. Future studies could examine the effects of APC generated by modified SIS on endothelial cell signaling.
Cook Biotech; American Heart Association; National Institutes of Health; OHSU Vertex Pharmaceuticals Scholarship
Keywords:
biofunctionalization,
Bioactive molecule,
acellullar matrix,
hemocompatiblility
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials for cardiovascular applications, vascular grafts and embolic devices
Citation:
Glynn
JJ and
Hinds
MT
(2016). Bioactive modification of decellularized matrix for vascular applications.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01072
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Monica T Hinds, Oregon Health & Science University, Biomedical Engineering, Portland, OR, United States, hindsm@ohsu.edu