Introduction: Bone is a living tissue mainly populated with osteoblastic cells (OB), osteoclastic cells (OC) and endothelial cells (EC). Their crosstalk is essential for bone homeostasis and to optimize bone regeneration after biomaterial implantation. Whereas interactions between OB/OC and between OB/EC are well established, the communication between OC and EC remains unknown[1]. Moreover, no tri-culture system from human primary cells has been described[2]. In the present study, the fate of an original primary human tri-culture model was investigated. The osteoclastic activity, the osteoblastic and endothelial phenotypes were assessed.
Materials and Methods: The co-culture medium (αMEM/EGM2*) was made of equal volumes of αMEM and EGM2*. Mononuclear cells (MNC) were isolated from human umbilical cord blood. OC and EPC were obtained after MNC differentiation. Endothelial Progenitor Cells (EPC) were used as EC model. Mesenchymal stem cells were isolated from human bone marrow and differentiated towards OB. Osteoclastic differentiation was characterized by TRAP staining and activity was assessed on calcium phosphate substrates. Both in a single culture model and in a co-culture model, OB and EPC were characterized by ALP staining and immunolabellings of vWF, CD-31 and VE-cadherin, respectively. Mineralization of extracellular matrix was characterized by Von Kossa staining. In the tri-culture model OB, OC and EPC were cultured either in direct or in indirect contact (OB and EPC were cultured in a transwell whereas MNC remained on the plastic).
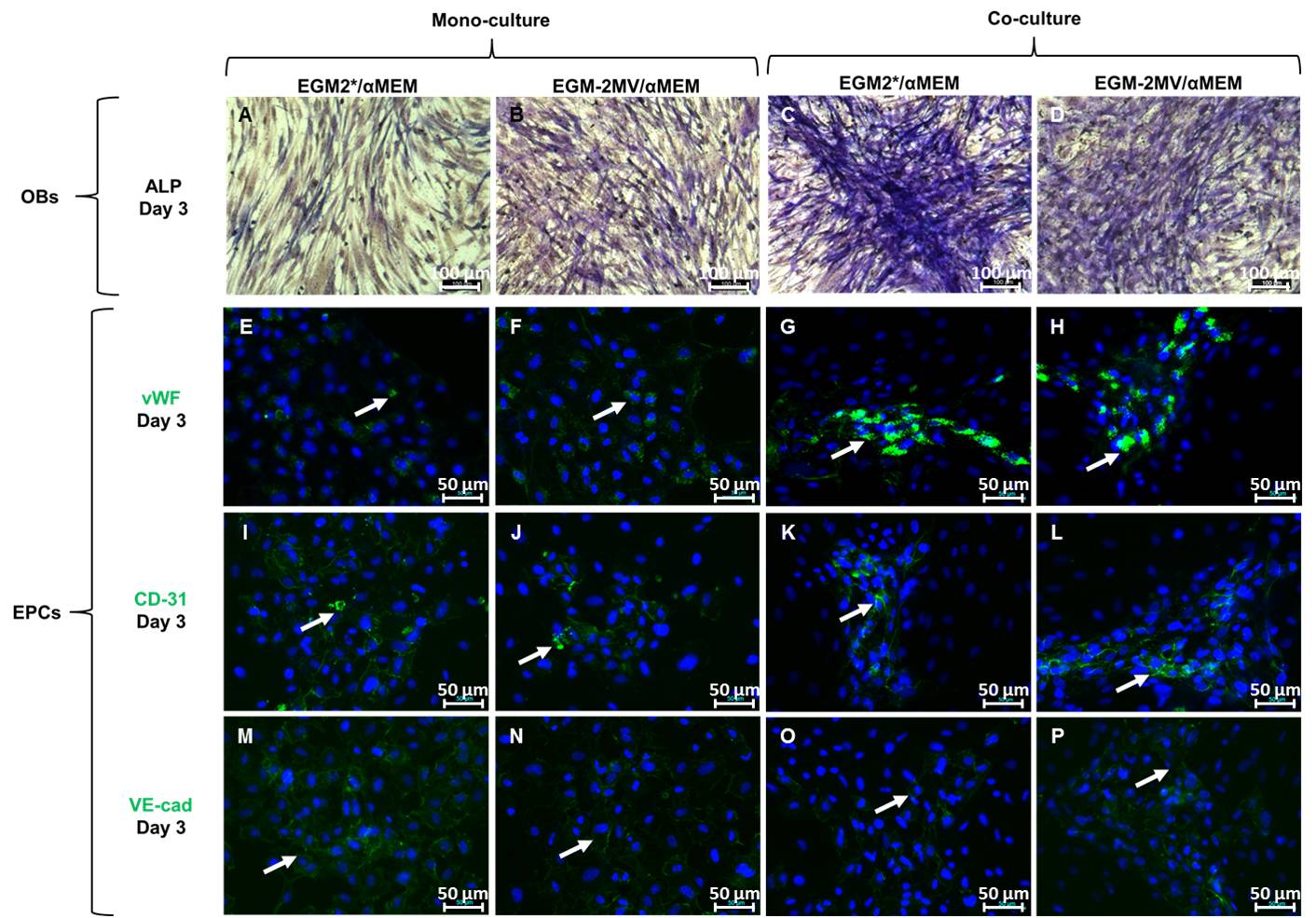
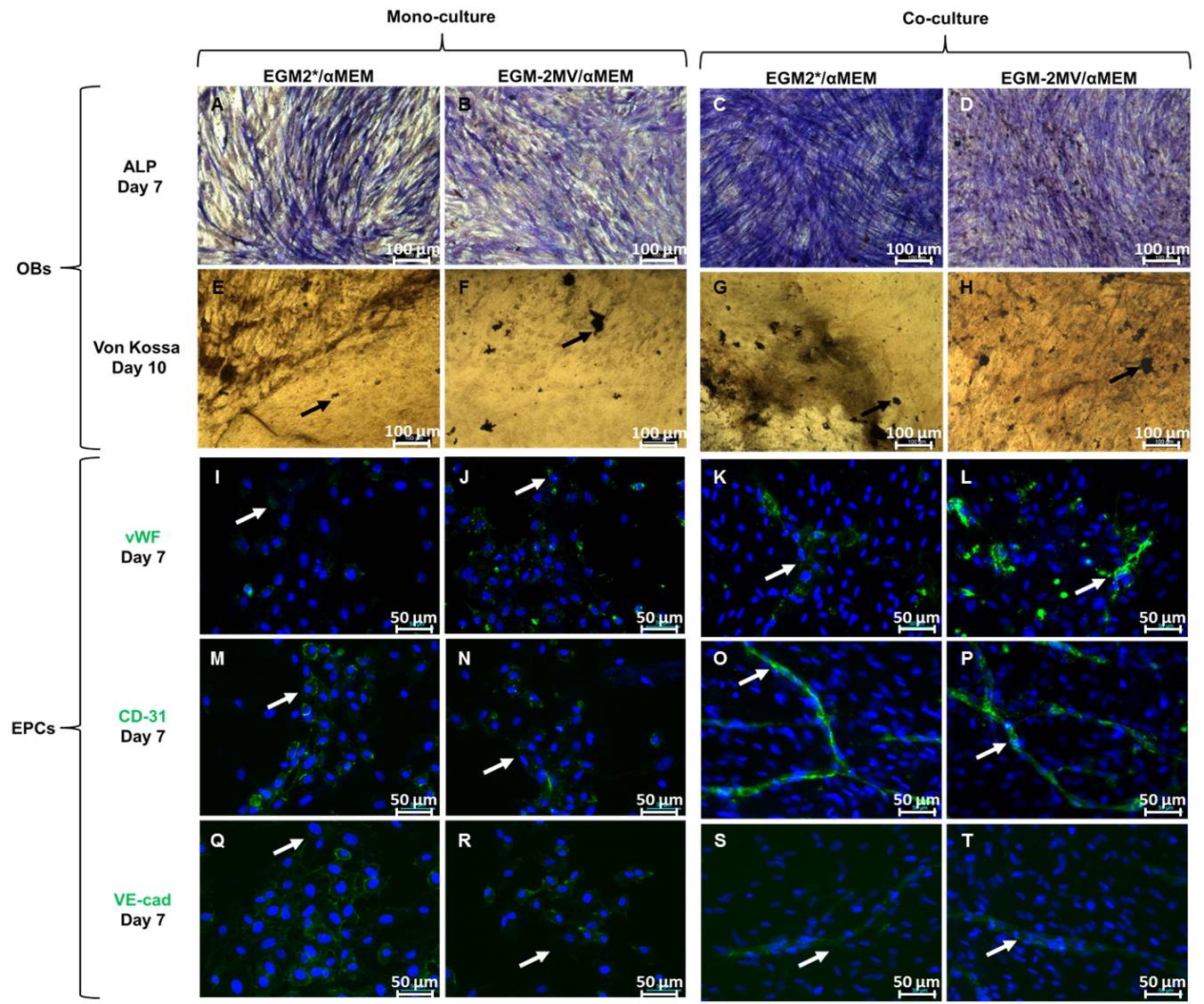
Results and Discussion: In all cell culture conditions, both immunolabellings of vWF, CD-31 and VE-cadherin as well as ALP expression and ECM mineralization were similar with αMEM/EGM2* compared with αMEM/EGM2-MV (control condition) and with αMEM/EGM2* compared with αMEM (control condition).
Moreover, osteoclastic resorptive activity was not significantly changed (5286 µm² ± 1150 µm² Vs. 4335 µm² ± 1104 µm², n=6, p=0.24).
The possibility of culturing the OB, OC and EPC together was then investigated. Mainly aiming for osteoclast formation, OB and OC markers were assessed after 11 days of culture. Both TRAP and ALP positive cells were observed. However, as previously published[3], EPC cannot be detected after more than 7 days of culture in a co-culture system. Interestingly, when the tri-culture was performed in indirect contact, a higher osteoclastogenesis was observed whereas osteoclast activity was not significant different in direct contact tri-culture system compare with control condition.
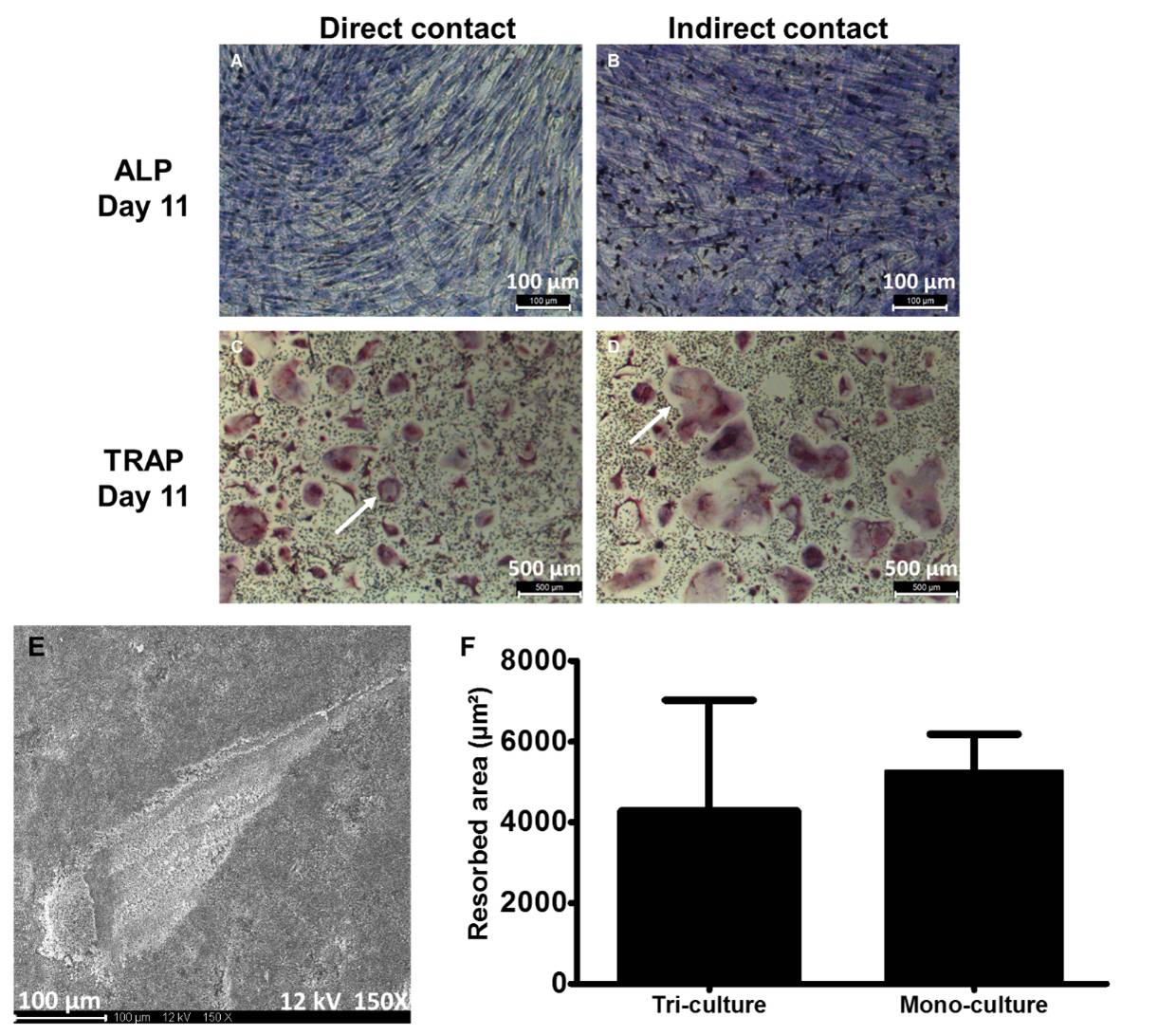
These data strongly suggested different kind of interactions between OB, OC and EPC in our models.
Conclusion: These overall results validate the use of αMEM/EGM2* as a medium to obtain functional OC, to preserve osteoblastic and endothelial phenotypes and finally to set up an original tri-cultured model. This model is a promising tool both to study bone cell-material interaction and to optimize strategies in bone tissue engineering.
Biomatlante for providing the calcium phosphate materials; The Marie Curie Carrier Integration Grant for their financial support
References:
[1] Thebaud N. et al., J. Tissue Eng Regen Med. 6:51-60, 2012.
[2] Dohle E. et al., European Cells and Materials. 27:149-165, 2014.
[3] Stahl A. et al., Biochem Biophys Res Commun 2004;322:684.