Biofunctionalization of degradable polar/hydrophobic/ionic polyurethanes with different fibronectin coatings: Study of monocyte behavior
-
1
University of Toronto, Institute of Biomaterials and Biomedical Engineering (IBBME) and the Faculty of Dentistry, Canada
-
2
University of Cergy-Pontoise, Biomaterial for Health Group, ERRMECe, France
Introduction: A degradable-polar hydrophobic ionic polyurethane (D-PHI) was developed to promote an anti-inflammatory monocyte response and wound healing[1],[2]. Biofunctionalization of D-PHI with fibronectin (Fn) can generate a biomimetic substrate of extracellular-matrix-like nature to enhance cell function within its environment. The current work aims to study the influence of the intrinsic D-PHI material chemistry[3] on Fn adsorption, and to investigate the influence of the Fn surface modification on the activity of human monocytes.
Materials and Methods: The synthesis of D-PHI surfaces was carried out by radical polymerization of an in-house divinyl oligomer (DVO), methacrylic acid (MAA) and methyl methacrylate (MMA) with stoichiometric ratios of DVO : MAA : MMA[2], of 1:5:15 for original D-PHI, 1:1:7 for LHLI, and 1:20:39 for HHHI[3]. Glass coverslips were used as a control surface. The biofunctionalization was generated using either coating of a monolayer Fn or adsorption of a multi layer-by-layer assembly consisting of (poly-L-lysine (PLL)-Fn)5. Surfaces were characterized by contact angle and adsorbed Fn was quantified by a bicinchoninic acid assay (BCA). Monocytes were isolated from whole human blood (U of T ethics approval #22203)[3],[4]. Cell morphology was examined by scanning electron microscopy (SEM), cellularity by DNA quantification, and metabolic activity by a WST assay.
Results and Discussion: The contact angle decreased with addition of a single Fn coating layer and increased with the (PLL-Fn)5 on polyurethanes but only showed increases for glass in both conditions (Table 1). Glass showed the greatest Fn adsorption for both mono and multilayers, whereas HHHI was differentiated from original D-PHI and LHLI, adsorbing higher Fn for monolayer but then lower for multilayers. DNA increased with the single Fn and PLL-Fn conditions on HHHI. Glass showed the most substantial change in DNA, with a 2-4 fold drop in DNA when Fn was present. Glass showed highest WST levels.
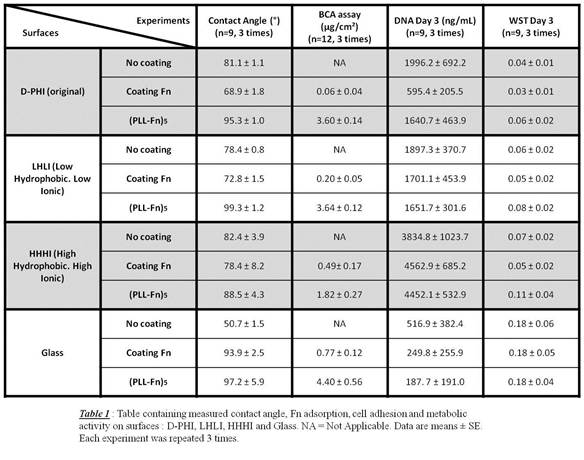
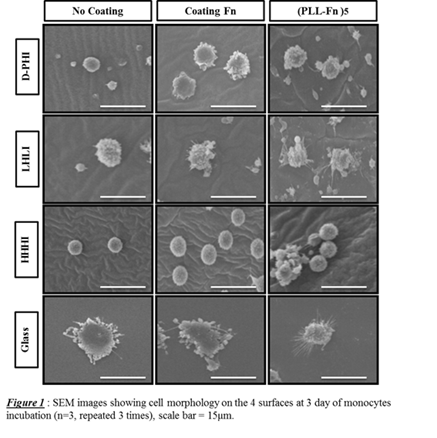
Fn increased cell activation (based on cell shape) for all surfaces except HHHI (Fig 1), where the latter enabled adhesion but did not activate extensively (i.e. very round cells), with/without Fn. TNFα and IL-10 were 4 fold higher on glass than on HHHI (data not shown) despite the inverse trends for DNA values (Table 1).
Conclusion: Chemical diversity in biomaterials is critical in terms of defining protein interactions and cell compatibility since Fn in single film vs multi-layer structures yielded very different interactions for polyurethanes synthesized from relatively small changes in hydrophobic and ionic distributions relative to their non-ionic polar content. This chemical diversity is further emphasized when homogeneous hydrophilic glass is compared to all three of the chemically diverse D-PHI formulations. On-going work is investigating the molecular mechanisms of monocyte response with respect to Fn molecule presentation the cells interacting with the different Fn and multi-layer films.
Natural Sciences and Engineering Research Council Synergy grant
References:
[1] Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 2008;20:86-100.
[2] Sharifpoor S, Labow RS, Santerre JP. Synthesis and Characterization of Degradable Polar Hydrophobic Ionic Polyurethane Scaffolds for Vascular Tissue Engineering Applications. Biomacromolecules 2009;10:2729-2739.
[3] Battiston KG, Ouyang B, Honarparvar E, Qian J, Labow RS, Simmons CA, et al. Interaction of a block-co-polymeric biomaterial with immunoglobulin G modulates human monocytes towards a non-inflammatory phenotype. Acta Biomaterialia 2015;24:35-43.
[4] Battiston KG, Labow RS, Santerre JP. Protein binding mediation of biomaterial-dependent monocyte activation on a degradable polar hydrophobic ionic polyurethane. Biomaterials 2012;33:8316-8328.
Keywords:
biomaterial,
protein,
biofunctionalization,
Cell response
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
General Session Oral
Topic:
Protein interactions with biomaterials
Citation:
Gossart
A,
Battiston
KG,
Gand
A,
Pauthe
E and
Santerre
J
(2016). Biofunctionalization of degradable polar/hydrophobic/ionic polyurethanes with different fibronectin coatings: Study of monocyte behavior.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01040
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Audrey Gossart, University of Toronto, Institute of Biomaterials and Biomedical Engineering (IBBME) and the Faculty of Dentistry, Toronto, ON, Canada, Email1
Dr. Kyle G Battiston, University of Toronto, Institute of Biomaterials and Biomedical Engineering (IBBME) and the Faculty of Dentistry, Toronto, ON, Canada, Email2
Dr. Adeline Gand, University of Cergy-Pontoise, Biomaterial for Health Group, ERRMECe, Cergy-Pontoise, France, adeline.gand@u-cergy.fr
Dr. Emmanuel Pauthe, University of Cergy-Pontoise, Biomaterial for Health Group, ERRMECe, Cergy-Pontoise, France, Email3
Dr. J.paul Santerre, University of Toronto, Institute of Biomaterials and Biomedical Engineering (IBBME) and the Faculty of Dentistry, Toronto, ON, Canada, Email4