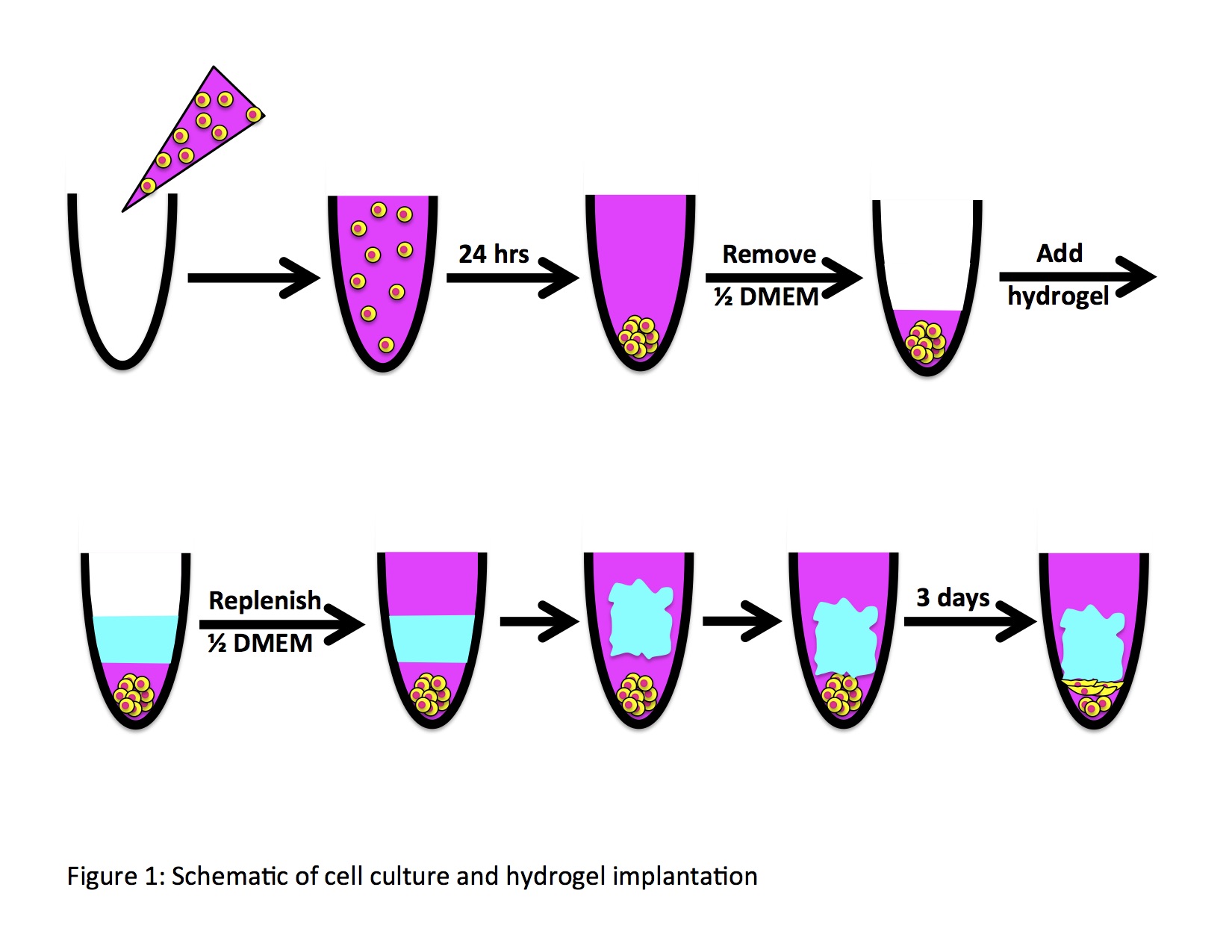
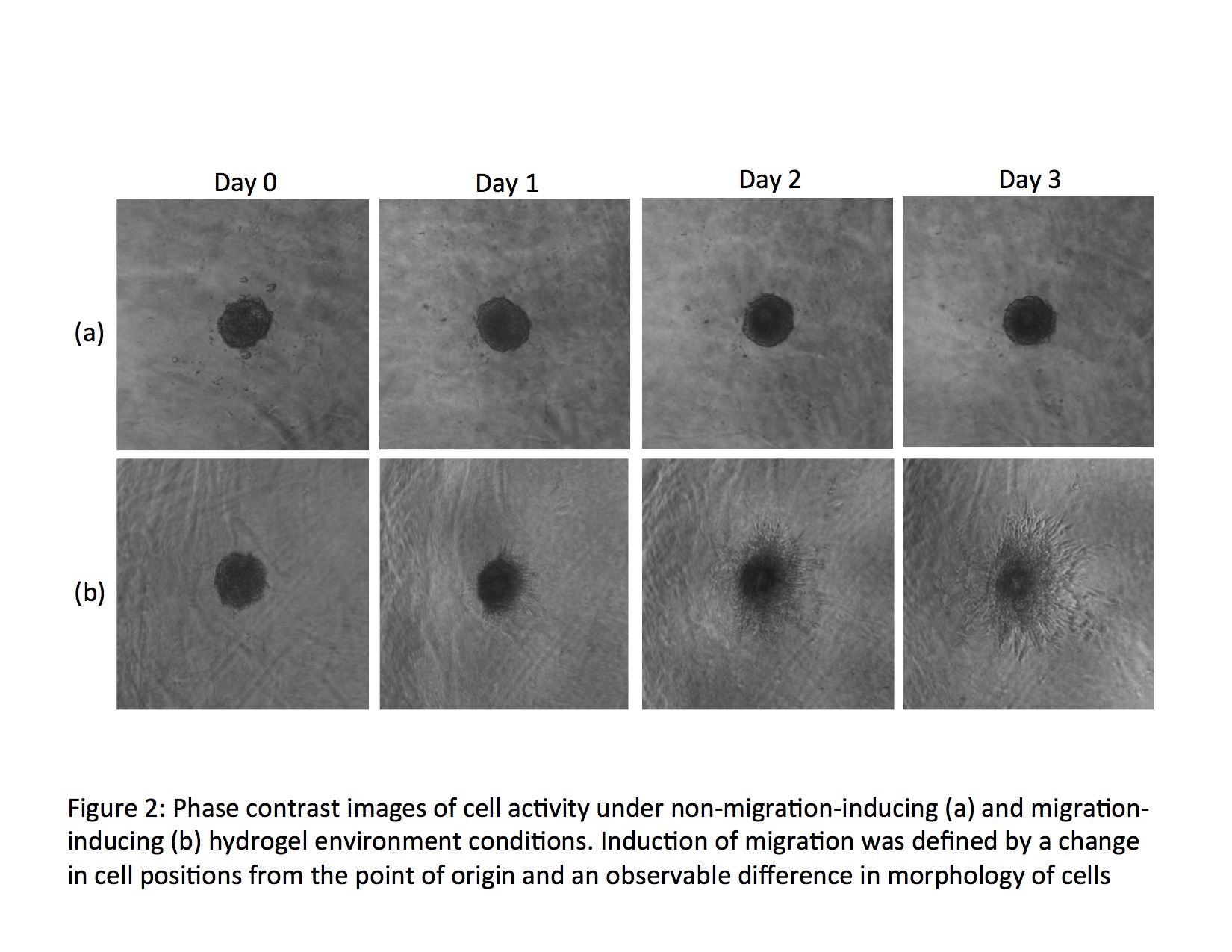
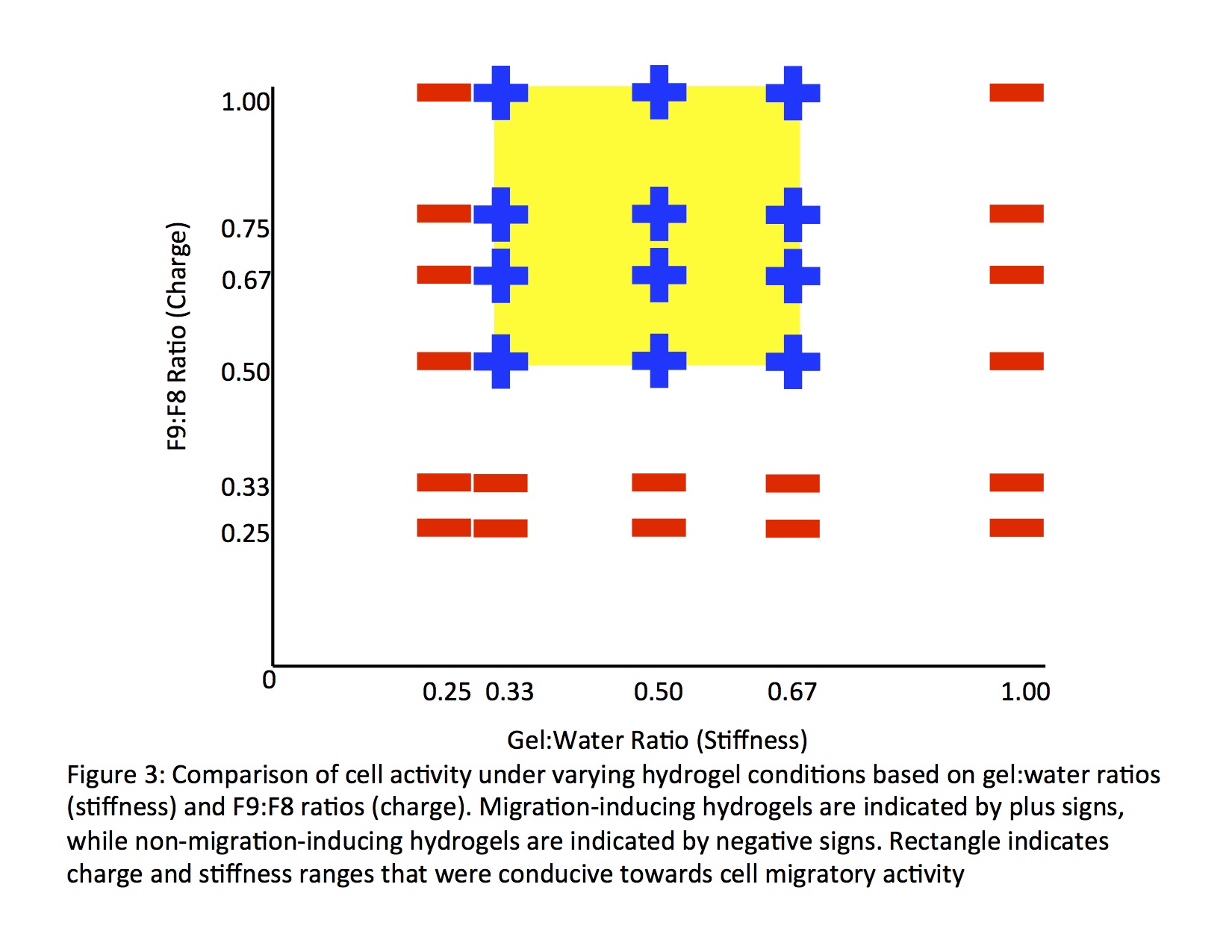
Self-assembling peptide-based hydrogels can serve as a versatile platform for a variety of biological applications. The wide range of possibilities for modulation of the properties of these hydrogels allow this platform to be broadly applied to a multitude of cell-based assays based on the specific demands of the system. Here, we demonstrate this flexibility of a self-assembling peptide-based hydrogel system by determining the effects of stiffness, charge, and arginylglycylaspartic acid (RGD) content of the hydrogels used in a cell-based culture and how these factors can be manipulated to maximize and promote cell proliferation. NIH 3T3 fibroblast cells were seeded as a cell spheroid and the hydrogel was added to the culture as a free-standing entity. Through this study, an optimum range of stiffnesses and a minimum viable charge were defined for the surrounding hydrogel environment of the fibroblasts for their proliferative growth, while the infusion of RGD in hydrogels allowed for proliferative activity outside of the charge ranges of hydrogels without the sequence. This evaluation of in vitro conditions for a specific cell type and platform can be applied to a broad scope of cell-based assays to study the proliferation and expansion of cells under externally applied experimental conditions. The versatility in the synthesis of an in vitro assay can allow for its use in various ranges of applications that depend on studying the effects of external environments on cell proliferation. This provides an effective means of developing in vitro assays for application in research dependent on a firm understanding of the effect of many external conditions in a cell-based platform, making it possible to extend these principles to promote advances in immune, microbiological, and oncological research and pharmacology.
David Belair; Vicki Workman
References:
[1] Boothroyd, Stephen, Aline F. Miller, and Alberto Saiani. "From Fibres to Networks Using Self-assembling Peptides." Faraday Discuss. Faraday Discussions 166 (2013): 195.
[2] Collier, Joel H., Jai S. Rudra, Joshua Z. Gasiorowski, and Jangwook P. Jung. "Multi-component Extracellular Matrices Based on Peptide Self-assembly." Chemical Society Reviews Chem. Soc. Rev. 39.9 (2010): 3413.
[3] Diaz, L. A. Castillo, A. Saiani, J. E. Gough, and A. F. Miller. "Human Osteoblasts within Soft Peptide Hydrogels Promote Mineralisation in Vitro." Journal of Tissue Engineering 5.0 (2014).
[4] Haspel, Howard C., Gloria M. Scicli, Gerald McMahon, and A. Guillermo Scicli. "Inhibition of Vascular Endothelial Growth Factor-Associated Tyrosine Kinase Activity with SU5416 Blocks Sprouting in the Microvascular Endothelial Cell Spheroid Model of Angiogenesis." Microvascular Research 63.3 (2002): 304-15.
[5] Jonker, Anika M., Dennis W. P. M. Löwik, and Jan C. M. Van Hest. "Peptide- and Protein-Based Hydrogels." Chemistry of Materials Chem. Mater. 24.5 (2012): 759-73.
[6] Kim, Choong, Seok Chung, Young Eun Kim, Kang Sun Lee, Soo Hyun Lee, Kwang Wook Oh, and Ji Yoon Kang. "Generation of Core-shell Microcapsules with Three-dimensional Focusing Device for Efficient Formation of Cell Spheroid." Lab Chip 11.2 (2011): 246-52.
[7] Korff, Thomas, and Hellmut G. Augustin. "Integration of Endothelial Cells in Multicellular Spheroids Prevents Apoptosis and Induces Differentiation." The Journal of Cell Biology 143.5 (1998): 1341-352.
[8] Korff, Thomas, and Hellmut G. Augustin. "Tensional Forces in Fibrillar Extracellular Matrices Control Directional Capillary Sprouting." Journal of Cell Science(1999): 3249-258.
[9] Kretsinger, Juliana K., Lisa A. Haines, Bulent Ozbas, Darrin J. Pochan, and Joel P. Schneider. "Cytocompatibility of Self-assembled β-hairpin Peptide Hydrogel Surfaces." Biomaterials 26.25 (2005): 5177-186.
[10] Kunz-Schughart, L. A., Freyer, J. P., Hofstaedter, F., and Ebner, R. "The Use of 3-D Cultures for High-Throughput Screening: The Multicellular Spheroid Model." Journal of Biomolecular Screening 9.4 (2004): 273-85.
[11] Lin, Ruei-Zeng, and Hwan-You Chang. "Recent Advances in Three-dimensional Multicellular Spheroid Culture for Biomedical Research." Biotechnol. J. Biotechnology Journal 3.9-10 (2008): 1285.
[12] Loo, Yihua, Shuguang Zhang, and Charlotte A.E. Hauser. "From Short Peptides to Nanofibers to Macromolecular Assemblies in Biomedicine." Biotechnology Advances 30.3 (2012): 593-603.
[13] Maude, Steven, Eileen Ingham, and Amalia Aggeli. "Biomimetic Self-assembling Peptides as Scaffolds for Soft Tissue Engineering." Nanomedicine 8.5 (2013): 823-47.
[14] Mujeeb, Ayeesha, Aline F. Miller, Alberto Saiani, and Julie E. Gough. "Self-assembled Octapeptide Scaffolds for in Vitro Chondrocyte Culture." Acta Biomaterialia 9.1 (2013): 4609-617.
[15] Roberts, D., C. Rochas, A. Saiani, and A. F. Miller. "Effect of Peptide and Guest Charge on the Structural, Mechanical and Release Properties of β-Sheet Forming Peptides." Langmuir 28.46 (2012): 16196-6206.
[16] Stahl, Andreas, Xiao Wu, Andreas Wenger, Michael Klagsbrun, and Peter Kurschat. "Endothelial Progenitor Cell Sprouting in Spheroid Cultures Is Resistant to Inhibition by Osteoblasts: A Model for Bone Replacement Grafts." FEBS Letters 579.24 (2005): 5338-342.
[17] Sutherland, R. "Cell and Environment Interactions in Tumor Microregions: The Multicell Spheroid Model." Science 240.4849 (1988): 177-84.
[18] Yan, Congqi, and Darrin J. Pochan. "Rheological Properties of Peptide-based Hydrogels for Biomedical and Other Applications." Chemical Society Reviews Chem. Soc. Rev. 39.9 (2010): 3528.
[19] Zhou, Mi, Andrew M. Smith, Apurba K. Das, Nigel W. Hodson, Richard F. Collins, Rein V. Ulijn, and Julie E. Gough. "Self-assembled Peptide-based Hydrogels as Scaffolds for Anchorage-dependent Cells." Biomaterials 30.13 (2009): 2523-530.