Introduction: Hydrogels made of biopolymers are promising for biomedical applications due to their good biocompatibility and intrinsic bioactivities1. Most of these biopolymer-based hydrogels are covalently crosslinked and are stable and stiff. However, these hydrogels are brittle and lack self-healing capability[1],[2]. Alternatively, supramolecular hydrogels stabilized by physical crosslinking, possess unique physical properties[3],[4]. However, to form these supramolecular hydrogels, chemical modifications of the biopolymers are still usually required, which may alter the properties of the biopolymers[5]. Herein, we show the formation of supramolecular hydrogels, free from chemical modifications and direct crosslinking of the biopolymers, via a novel “Host-Guest Macromer” (HGM) approach. This simple but effective strategy opens up a new route to develop biopolymeric supramolecular hydrogels with enhanced physical and biological functionalities as drug and/or cell carriers for regenerative medicine.
Materials and Methods: Gelatin HGM supramolecular hydrogels were prepared by pre-assembling acrylated host (β-cyclodextrin) monomer and gelatin (bearing guest aromatic groups) before radical chain polymerization. Stromal cell-derived factor (SDF-1α) was used for migration test. Hydrogels were soaked in dexamethasone (Dex) solutions to measure the percentage of Dex loaded into the hydrogels and then were incubated in PBS to measure the Dex releasing. Human mesenchymal stem cells (hMSCs) laden hydrogels were cultured in osteogenic media for in vitro study. Rat calvarial defect model was used for in vivo study.
Results and Discussion: The HGM gelatin hydrogels show outstanding tensile and compression properties (Fig. 1a, b). Moreover, our HGM hydrogels can be drawn into a syringe and injected into a mold of a different shape and recover to self-standing hydrogels (Fig. 1c). Furthermore, the HGM hydrogels bond the two pieces of bone tightly (Fig. 1d) even in the aqueous environment. Besides, HGM hydrogels facilitate the migration of hMSCs inside the hydrogels (Fig. 1e). This property can support the recruitment of endogenous cells to participate in the healing process. Compared to chemical crosslinking methacrylated gelatin (MeGel) hydrogesl, the HGM hydrogels hold more hydrophobic small molecular drug (Dex) and release it continuously for a longer time. For in vitro, our HGM hydrogels can support the growth and osteogenic differentiation of hMSCs (Fig. 2a-c). Finally, the implantation of the HGM gelatin hydrogels without any growth factors and cells in rat calvarial bone defects shows that our HGM hydrogels have the potential to modulate macrophage polarization and to support in situ tissue regeneration without using exogenous cells (Figure 2 d-e), thereby greatly simplifying the regenerative therapy.
Conclusion: Collectively, Our work provides an original “host-guest macromer” approach to prepare supramolecular hydrogels. The HGM hydrogels are ideal biomaterial to assist the repair and regeneration of injured tissues such as bone.
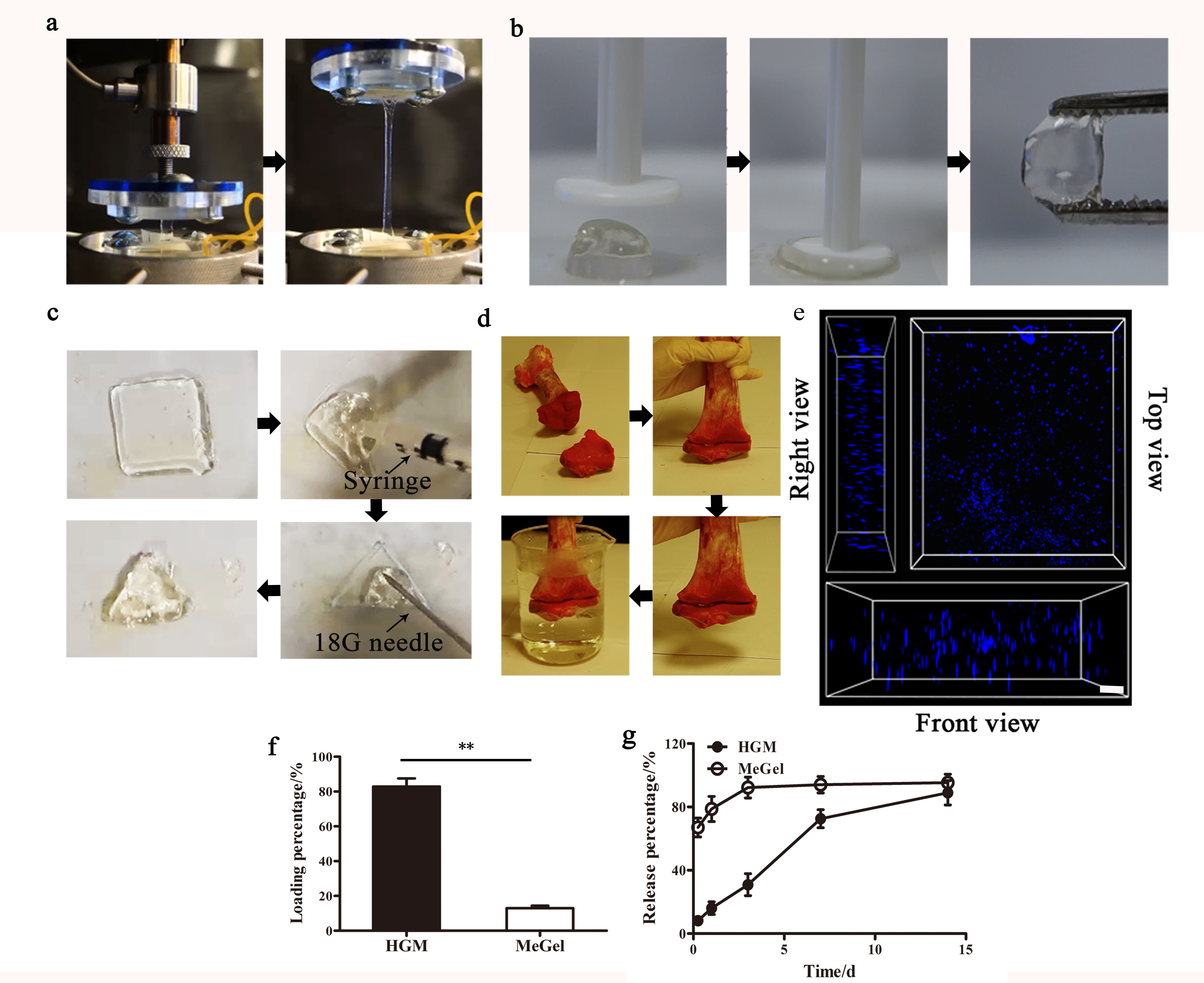
Figure 1. The unique features of (a) strechability, (b) compressibility, (c) injectability, (d) moldability, (e) tissue adhesion, (e) cell migration and drug loading (f) / realsing (g) of the HGM hydrogels. **p < 0.001.
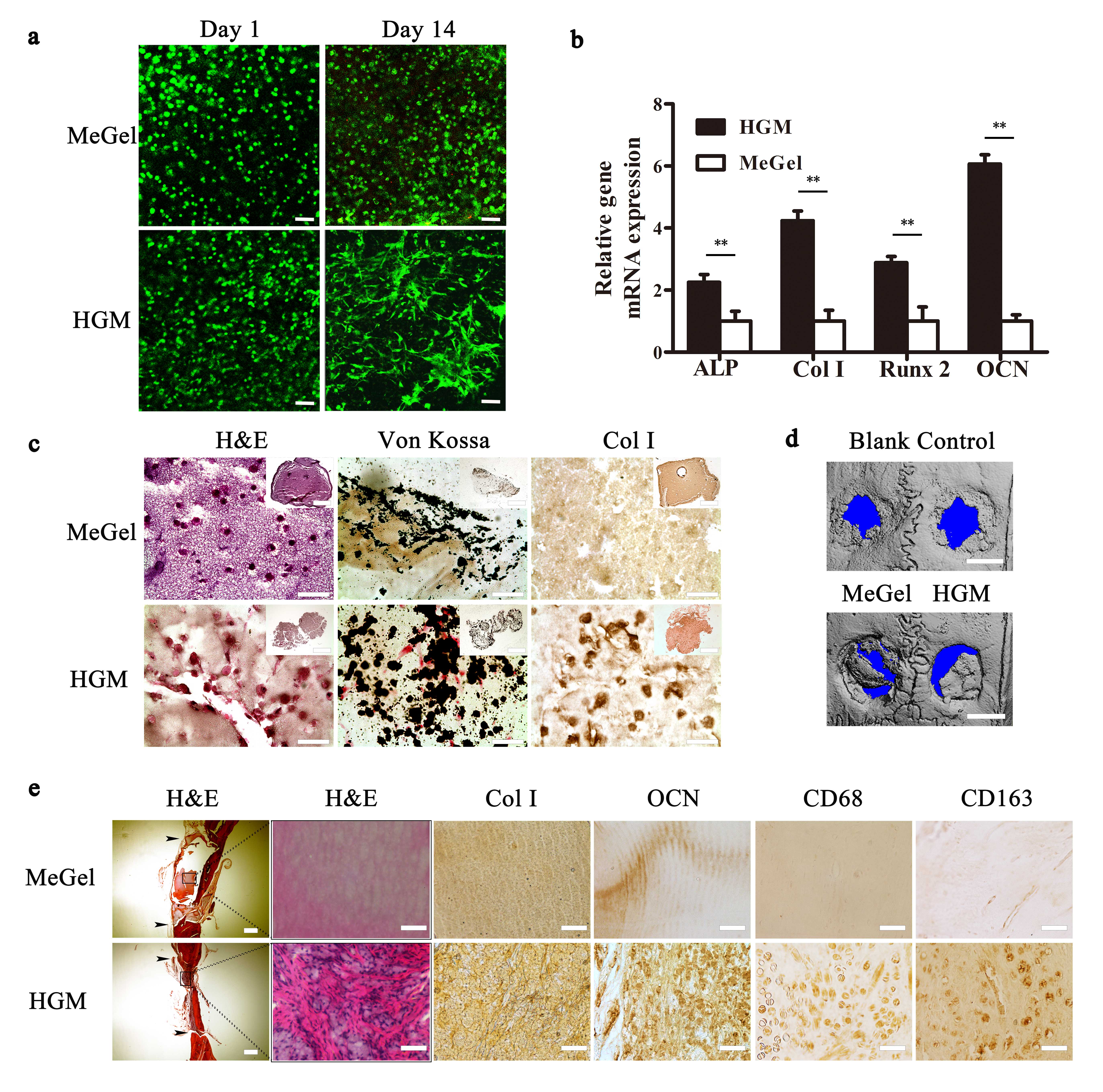
Figure 2. (a) Cell viability staining of the hMSC-laden MeGel and HGM hydrogels. (b) Selected osteogenic gene expression. (c) H&E staining, Von Kossa staining, and type I collagen immunohistochemical staining of hMSC-laden HGM and MeGel hydrogels after 14 days of osteogenic culture. (d) representative mCT reconstitution for blank group (without hydrogel), and Dex-loaded MeGel (left) and HGM (right) hydrogel. (e) H&E staining, Col I, osteocalcin (OCN), CD68, and CD163 immunohistochemical staining of the MeGel and HGM hydrogel groups after 10 weeks of implantation (black narrow: the calvarial defect boundary). Scale bars: 100 m (a), 50 m (c, and 100 m (inserts), 50 m (e, except the leftmost column of H&E staining, 500 m). **p < 0.001.
Research Grants Council of Hong Kong (Project No. 439913); National Natural Science Foundation of China (Project No. 31300796); Shun Hing Institute of Advanced Engineering (Project No. BME-8115043)
References:
[1] Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 336, 1124-1128, doi:10.1126/science.1214804 (2012).
[2] Balakrishnan, B. & Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 111, 4453-4474, doi:Doi 10.1021/Cr100123h (2011).
[3] Harada, A., Kobayashi, R., Takashima, Y., Hashidzume, A. & Yamaguchi, H. Macroscopic self-assembly through molecular recognition. Nat Chem 3, 34-37, doi:http:10.1038/NCHEM.893( 2011).
[4] Kakuta, T. et al. Preorganized hydrogel: self-healing properties of supramolecular hydrogels formed by polymerization of host-guest-monomers that contain cyclodextrins and hydrophobic guest groups. Advanced materials 25, 2849-2853, doi:10.1002/adma.201205321 (2013).
[5] Bigi, A., Cojazzi, G., Panzavolta, S., Rubini, K. & Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 22, 763-768, doi:http://dx.doi.org/10.1016/S0142-9612(00)00236-2 (2001).