Introduction: The conventional imaging method for bone repair is based on X-ray. Its main disadvantage is the presence of ionizing radiations. MRI appears as a non-invasive non-ionizing technique well suited to in vivo longitudinal evaluation of tissue repair following grafting procedure. Contrarily to biomaterials, cortical bone exhibits very short T2 relaxation time [1]. To measure biomaterial degradation, the balanced Steady-State Free Precession (bSSFP) sequence was employed as it can be used in 3D in short acquisition time, induces high Signal-to-Noise Ratio and allows to detect biomaterials with hyper intense signal. However, adipose tissue, main component of the bone marrow, induces similar signal that could hamper the detection of the biomaterials. In addition, inherent banding artifacts through the images are problematic. The goal of this study was to develop a MR sequence without fat signal or banding artifact at high magnetic field in order to repetitively survey volumes of biomaterials in bone defects.
Methods: Biomaterials were implanted into a defect within rat femoral condyles. Longitudinal MRI was performed 1, 3 and 5 weeks after implantation at 7T. After each time point, bones were extracted to perform CT scans.
A 3D bSSFP sequence was used in which the usual RF pulse was replaced by a water frequency-selective binomial pulse [2] containing 5 hermite sub-pulses of 150μs each, which intensities followed the schematic 1-2-3-2-1 (interpulse delay of 200μs). The slice selection gradient was removed. The other sequence parameters were: TE/TR=1.9/5.1ms; reception bandwidth =75kHz; FOV=30x35x25; matrix=192x192x128; 4 phase offsets; Flip Angle=27°; 2 averages; acquisition time=4min15s. The 3D images were reconstructed after a “Sum-Of-Square” (SOS) of the 4 offsets [3].
The 3D micro-CT scans parameters were: 15 μm resolution; 900 X-ray radiographs, source voltage=80 kV, current=80 mA, exposure time=3s.
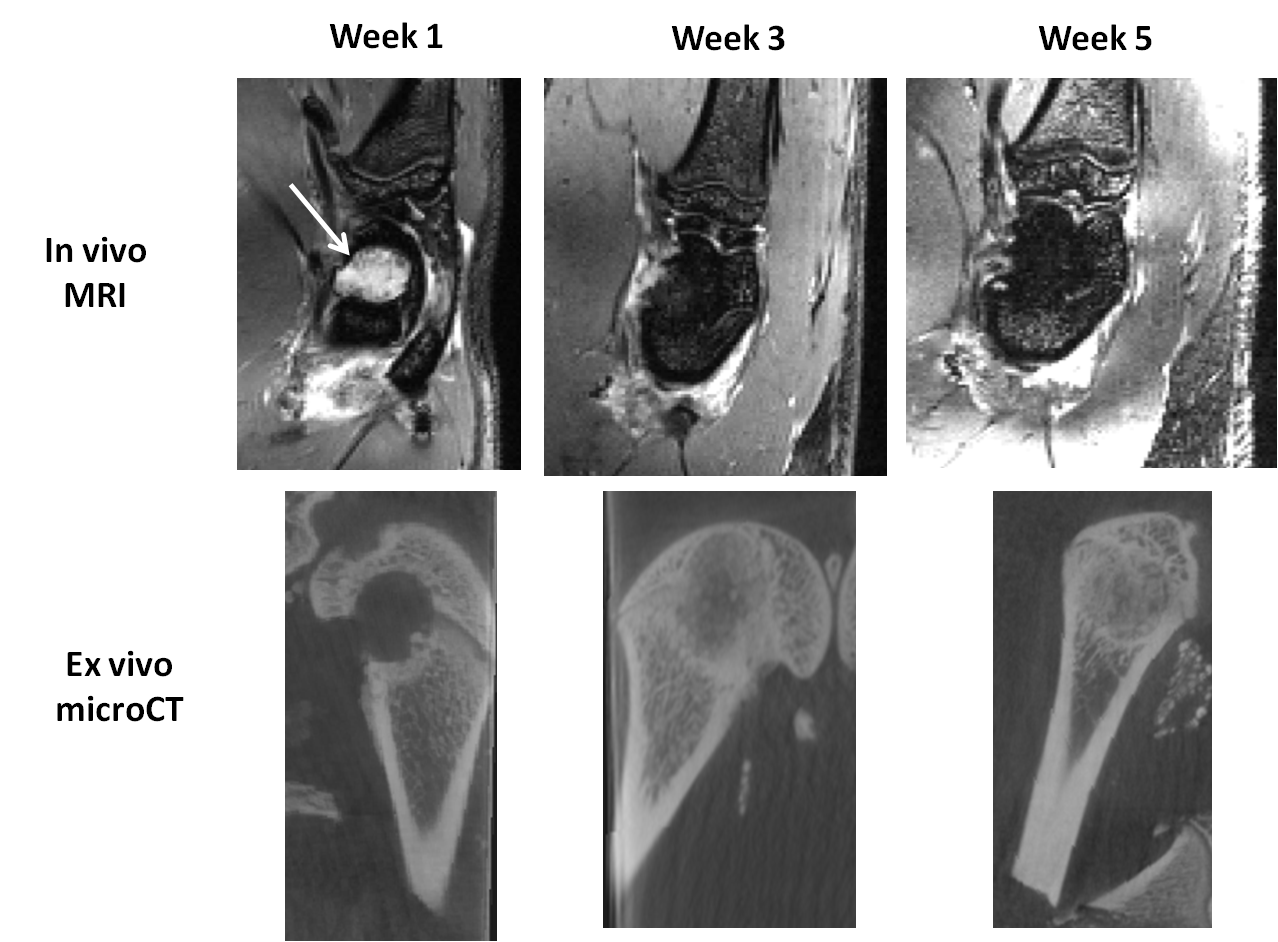
Results: The binomial pulse induced a drop in fat signal located subcutaneously and also within the bone marrow in the entire 3D FOV. The SOS reconstruction allowed to suppress all the banding artifacts caused by inhomogeneities in the magnetic field. Thus, the biomaterials were easily detected as hyper-intense areas within the bone marrow (arrow). While the biomaterial hyper-intense signal disappeared slowly over time, bone regeneration took place as demonstrated by the hypo-intense signal appearing within the bone defect. This degradation has been correlated with cortical bone formation observed with micro-CT.
Discussion: The 3D water-selective bSSFP sequence allowed to obtain high-resolution images without ionizing radiations. The degradation of the implanted biomaterials were followed longitudinally.
Conclusion: This study confirms the efficiency of MRI to monitor integration of a biomaterial in a bone site.
References:
[1] Schlaubitz S, et al. PLoS ONE 2014
[2] Yuan J, et al. J Magn Reson 2011
[3] Bangerter NK, et al. Magn Reson Med 2004