Introduction: Breast cancer (BC) is the most common cause of cancer-related mortality among women worldwide for the development of matastasis, mainly in bone and visceral sites[1],[2]. The interplay between tumor and stromal cells occurring in bone metastasis has not been deeply dissected due to the low availability of such clinical specimens. For this reason in vitro 3D models represent valid tools to investigate the biological pathways undergoing metastasis formation. In this work, we propose a polyurethane scaffold to study the interaction between patient-derived adipose derived stem cells (ADSCs), differentiated in osteoblasts, and breast cancer stem cells (BCSC).
Materials and Methods: Polyurethane foams (PU) and foams loaded with tricalcium phosphate (PU_L, 40% wCaP/wpolyol) were synthesised by a one-step gas foaming reaction, by stirring a poly-ether-polyol mixture, isocyanate MDI prepolymer, Fe-AcetylAcetonate as catalyst and 2% w/wpolyol water as expanding agent. Samples were observed by SEM to assess their morphology; compression mechanical tests were performed in wet condition. An in vitro preliminary study was carried out using osteoblast-like SAOS-2 cells to evaluate the scaffold ability to induce osteoblast activity. Viability and alkaline phosphatase (ALP) activity were assessed after 7 and 14 days of culture. The suitability of scaffolds as bone metastatic in vitro models was assessed seeding ADSC, induced to differentiate in osteoblasts using osteogenic medium for 4 weeks, subsequently co-cultured with BCSC for 2 weeks. Alizarin red was used to verify differentiation. Cell morphology and deposition of inorganic ECM by differentiated ADSC was investigated by SEM. BCSC proliferation and interaction with microenvironment was also evaluated. Data were compared by ANOVA.
Results and Discussion: Scaffold SEM images showed about 90% open porosity.

Mechanical properties (E, σmax) of PU and PU_L were significantly different (Figure 1): higher E and σmax of PU_L confirmed the reinforcement of CaP loading.
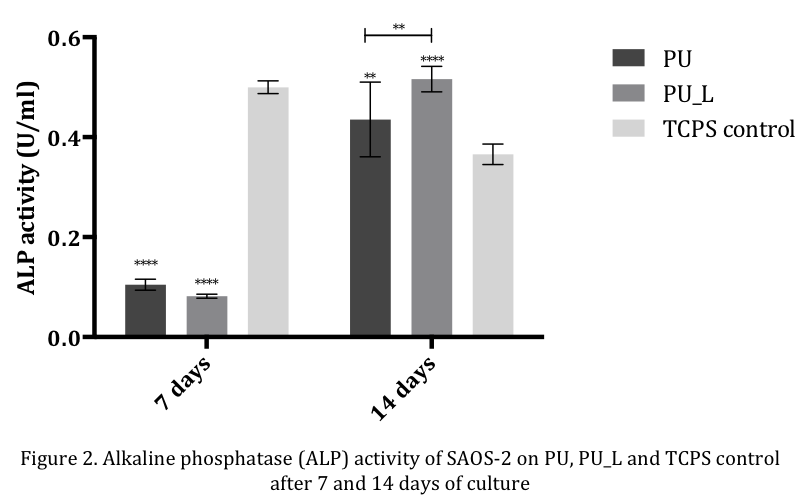
Viability of SAOS-2 increased until day 7 on both scaffolds, with a subsequent plateau phase. After 14 days of culture, SAOS-2 ALP activity was significantly higher on PU_L compared to TCPS control and PU, proving that higher stiffness and the presence of CaP particles promote osteoblast activity (Figure 2).

On both scaffolds ADSCs were able to adhere and differentiate (Figure 3); alizarin red showed inorganic ECM deposition after 4 weeks; SEM showed the co-cultured BCSC to form cancer cells aggregates.
Conclusion: Scaffolds suitability as 3D bone metastasis microenvironment model was proved. We demonstrated that ADSC were able to adhere to the scaffold, deposit inorganic ECM, and that BCSC were able to adhere on the pre-seeded cells forming aggregates. Future tests will include biochemical assays to further characterize pathways involved in bone metastasis development.
References:
[1] Ca Cancer J Clin 2014; 64:9-29
[2] Adv Funct Mat 2013; 42:5249-5260