Introduction: Blindness and visual impairment are largely caused by irreversible loss of light sensitive photoreceptors in the retina. Transplantation of photoreceptor cells and their progeny is one of the most promising strategies to restore vision[1]. But, using conventional injection strategies, the distribution, survival and integration of such cells in vivo remains a major impediment to clinical applications. To enhance retinal cell distribution and survival in vivo, we designed an injectable hyaluronan-based hydrogel that met with much success[2]-[7]. Yet, 90% (or more) of transplanted cells are still dying. In order to make a more significant difference, further chemical modifications of HAMC with various pro-survival factors are being investigated to deliver the cells in a more conducive environment for even greater survival and integration.
Materials and Methods: A hydrogel formulation comprised of a physical blend of hyaluronan (HA) and methylcellulose (MC), HAMC, was developed and optimized for subretinal delivery. The injectable hydrogel was tested in vivo for transplantation of retinal cells and their photoreceptor progeny. HAMC was then chemically modified to allow thiol-maleimide and biotin–streptavidin chemistry, and conjugated with two pro-survival factors. The modified hydrogel is being tested with photoreceptor cells in vitro and in vivo.
Results and Discussion: The HAMC hydrogel is particularly well-suited for delivery into the subretinal space as it is minimally swelling, bioresorbable within one week and it gels rapidly on injection[5]-[7]. When delivered in HAMC, retinal stem cells and their photoreceptor progeny were well-distributed in the subretinal space after injection (see Figure 1), and significantly better than those injected using conventional saline strategies, where the cells aggregated together[2]-[4]. Cell survival was doubled from approximately 4% to 8% when delivered in HAMC vs. saline controls.
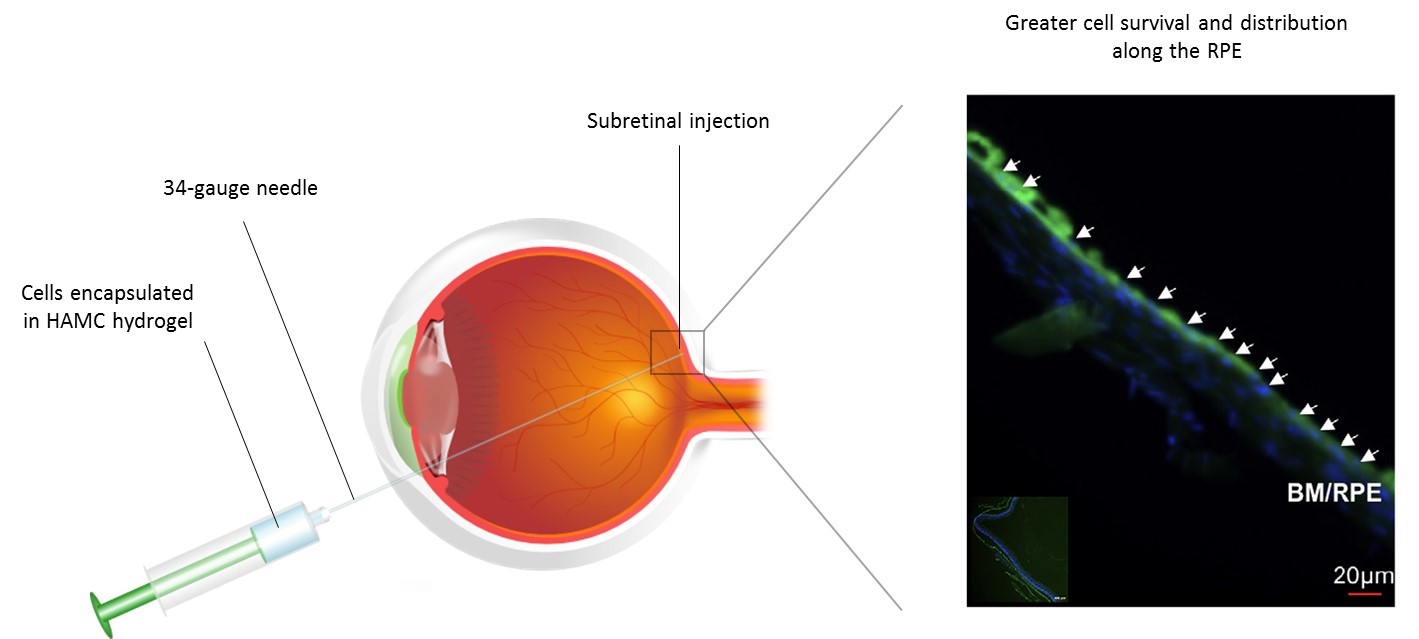
Figure 1. Delivery of retinal progenitor cells (RPCs) to the subretinal space, using hyaluronan/methylcellulose (HAMC) hydrogel as biomaterial support.
To further enhance the properties of this hydrogel, HAMC was then covalently modified with two pro-survival factors. The success of the synthesis was assessed by mass spectroscopy and amino acid analysis, prior to a complete study of photoreceptor cell viability first in vitro and then in vivo. The HAMC hydrogel, conjugated with pro-survival factors, should enhance both distribution and survival of transplanted retinal photoreceptor cells.
Conclusions: These data demonstrate that HAMC serves as a platform strategy for cell delivery, enabling minimally-invasive strategies and significantly better tissue and functional outcomes than controls.
B.G.B. holds a CIHR Doctoral Canada Graduate Scholarship and McLaughlin Centre Graduate Fellowship; M.J.C. holds a Mitacs Elevate post-doctoral fellowship and thanks the Stem Cell Network for financial support; This work was supported in part by the CIHR, the Heart and Stroke Foundation, the NIH (R01 EY015716), the Foundation Fighting Blindness (FFB) Canada/Krembil Foundation, the McEwen Centre for Regenerative Medicine, and the Ontario Research Foundation
References:
[1] V. Delplace, S. Payne, M. Shoichet, Delivery strategies for treatment of age-related ocular diseases: from a biological understanding to biomaterial solutions., J. Control. Rel., (2015).
[2] B.G. Ballios, M.J. Cooke, L. Donaldson, B.L. Coles, C.M. Morshead, D. van der Kooy, M.S. Shoichet, A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation, Stem Cell Reports, 4 (2015) 1031-1045.
[3] B.G. Ballios, M.J. Cooke, D. van der Kooy, M.S. Shoichet, A hydrogel-based stem cell delivery system to treat retinal degenerative diseases, Biomaterials, 31 (2010) 2555-2564.
[4] R.Y. Tam, T. Fuehrmann, N. Mitrousis, M.S. Shoichet, Regenerative therapies for central nervous system diseases: a biomaterials approach, Neuropsychopharmacology, 39 (2014) 169-188.
[5] H. Kim, M.J. Cooke, M.S. Shoichet, Creating permissive microenvironments for stem cell transplantation into the central nervous system, Trends Biotechnol., 30 (2012) 55-63.
[6] D. Gupta, C.H. Tator, M.S. Shoichet, Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord, Biomaterials, 27 (2006) 2370-2379.
[7] M.D. Baumann, C.E. Kang, C.H. Tator, M.S. Shoichet, Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury, Biomaterials, 31 (2010) 7631-7639.