Introduction: Despite an increasing number of hemostatic agents available[1], bleeding still accounts for roughly half of all trauma deaths in hospitals[2]. The natural coagulation system is usually effective at halting moderate bleeding, and thus creating new materials that mimic fibrin may lead to new hemostatic agents[3]. Unique properties of fibrin include its ability to polymerize as a propagating reactive front[3], and its strong adhesion to injured tissues[4], which is catalyzed by the transglutaminase coagulation factor XIIIa (FXIIIa)[5]. We hypothesized that adding a FXIIIa-crosslinkable material to clots would make them more adhesive. Here, we show that a well-characterized FXIIIa-crosslinkable material[6]-[9] can mimic fibrin to polymerize by spatial propagation and increase clot adhesion.
Materials and Methods: A glutamine-donating peptide substrate of FXIIIa was conjugated to PEG (Gln-PEG) and formulated with an amine-donor, spermidine, in plasma as described[10]. To measure frontal propagation, this mixture was flowed into tissue factor(TF)-patterned microfluidic channels[11] and polymerization was traced by time-lapse microscopy. To measure adhesion, a mix containing normal plasma (36%v/v), Gln-PEG (44mg/mL), spermidine (1.2mM), CaCl2 (4.8mM), NaCl (11mM), purified FXIII (0.18mg/mL), and APTT activator (2.4% v/v) was clotted between glass substrates. A lap-shear test was done using a mechanical analyzer (TA Q800).
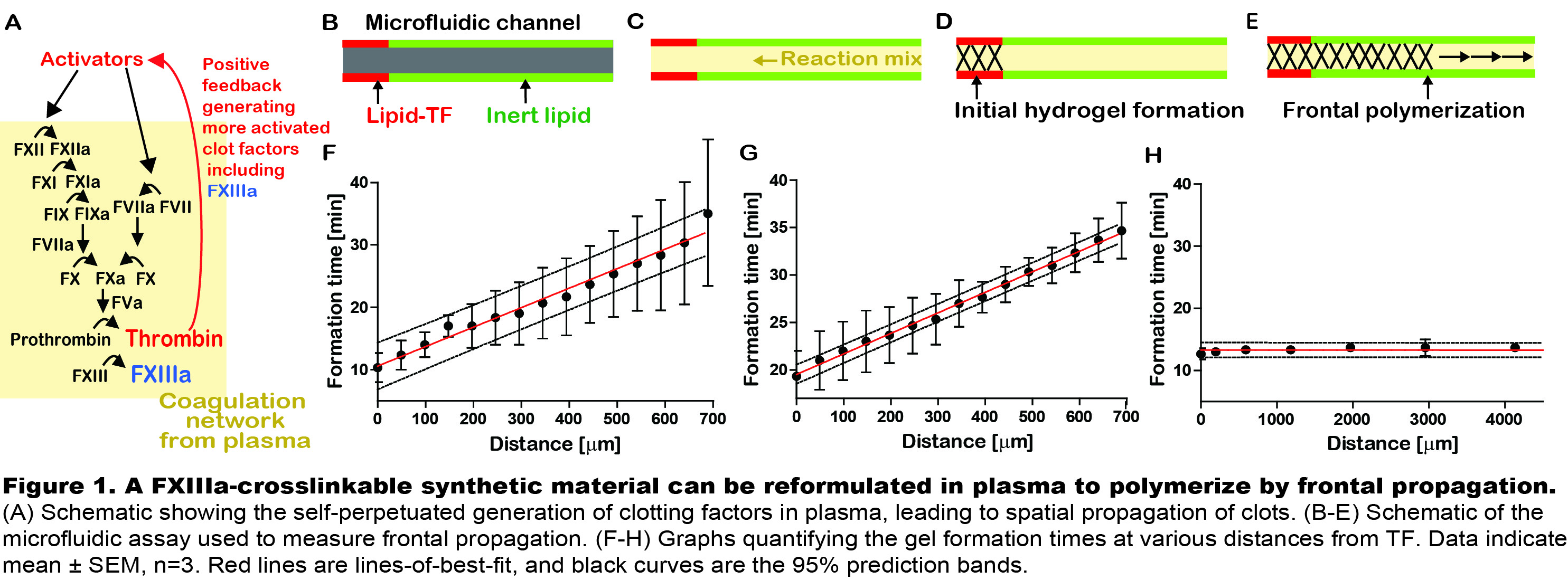
Results and Discussion: The self-assembly and polymerization of fibrin during clotting is a rare example of frontal polymerization in nature, where gelation spatially propagates as a reactive front[3]. To test whether a FXIIIa-crosslinkable synthetic material could mimic frontal polymerization of fibrin clots without flow, Gln-PEG and spermidine were added to fibrinogen(Fg)-deficient plasma in a microfluidic channel patterned with clot activator, TF (Fig.1A-E). Polymerization initiated at TF then propagated through stagnant plasma (32m/min, Fig.1F) similar to Fg-rich clots (21 m/min, Fig.1G). In contrast, when preactivated FXIIIa was added to Gln-PEG-spermidine, polymerization was uniform and did not propagate (Fig.1H). This shows that frontal polymerization in coagulation is dependent on the coagulation cascade, and a FXIIIa-crosslinkable synthetic material can mimic this unique polymerization property of fibrin.
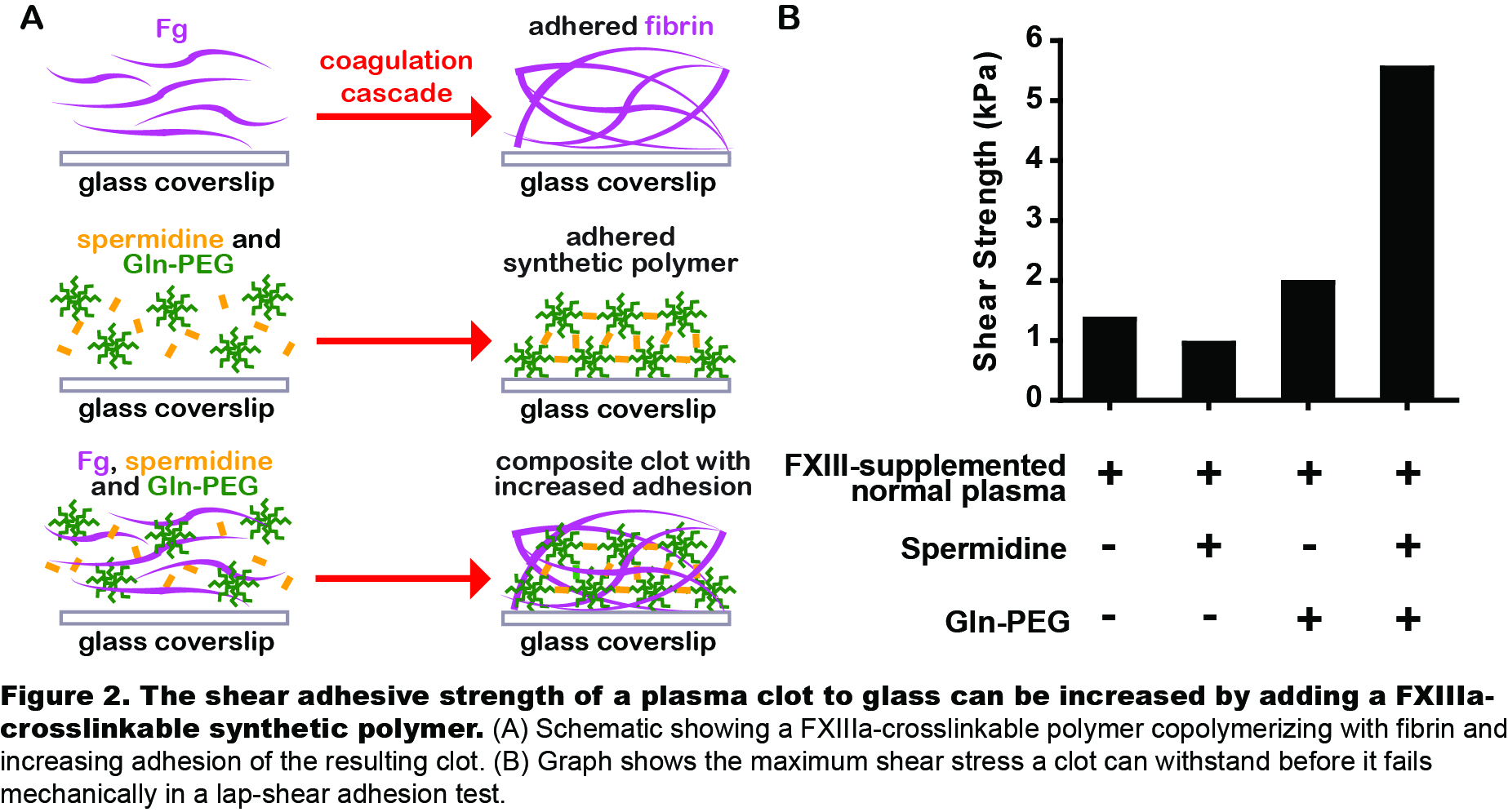
To test if adding a FXIIIa-crosslinkable material makes clots more adhesive, Gln-PEG and spermidine were added to plasma and the composite clot’s adhesion was measured in a lap-shear test (Fig.2A). Shear strength increased from 1.3kPa to 5.6kPa (Fig.2B). This shows a FXIIIa-crosslinkable synthetic material can supplement Fg to increase clot adhesion.
Conclusion: Coupling the polymerization of a synthetic material to the coagulation cascade allows it to mimic fibrin’s ability to form and adhere in response to signals of vascular injury. This strategy may be useful for developing novel hemostatic materials.
This work was funded by the Canadian Institutes of Health Research (MOP-119426 and MSH-130166), the Canadian Foundation for Innovation (31928), and the BC Knowledge Development Fund.
References:
[1] H.E. Achneck et al., "A Comprehensive Review of Topical Hemostatic Agents: Efficacy and Recommendations for Use," Annals of Surgery. Vol. 251, Feb. 2010.
[2] A. Sauaia et al., "Epidemiology of trauma deaths - a reassessment," Journal of Trauma. Vol. 38, Feb. 1995.
[3] M.K. Runyon, B.L. Johnson-Kerner, C.J. Kastrup, T.G. Van Ha and R.F. Ismagilov, "Propagation of Blood Clotting in the Complex Biochemical Network of Hemostasis Is Described by a Simple Mechanism," Journal of the American Chemical Society. Vol. 129, May 2007.
[4] J.B. Holcomb et al., "Fibrin Sealant Foam Sprayed Directly on Liver Injuries Decreases Blood Loss in Resuscitated Rats," Journal of Trauma. Vol. 49, May 2000.
[5] D.H. Sierra, "Fibrin Sealant Adhesive Systems: A Review of Their Chemistry, Material Properties and Clinical Applications," Journal of Biomaterials Applications. Vol. 7, Apr. 1993.
[6] V. Milleret, B.R. Simona, P.S. Linenemann, J. Voros and M. Ehrbar, "Electrochemical Control of the Enzymatic Polymerization of PEG Hydrogels: Formation of Spatially Controlled Biological Microenvironments," Advanced Healthcare Materials. Vol. 3, Apr. 2014.
[7] K.A. Mosiewicz* and L. Kolb* et al., "In situ cell manipulation through enzymatic hydrogel photopatterning," Nature Materials. Vol. 12, Nov. 2013.
[8] G. von Maltzahn et al., "Nanoparticles that communicate in vivo to amplify tumour targeting," Nature Materials. Vol. 10, Jun. 2011.
[9] T.J. Sanborn, P.B. Messersmith and A.E. Barron, "In situ crosslinking of a biomimetic peptide-PEG hydrogel via thermally triggered activation of factor XIII," Biomaterials. Vol. 23, Jul. 2002.
[10] J.H. Yeon* and K.Y.T. Chan* et al., "A biochemical network can control formation of a synthetic material by sensing numerous specific stimuli," Scientific Reports. Vol. 5, May 2015.
[11] C.J. Kastrup, M.K. Runyon, E.M. Lucchetta, J.M. Price and R.F. Ismagilov, "Using chemistry and microfluidics to understand the spatial dynamics of complex biological networks," Accounts of Chemical Research. Vol. 41, Jan. 2008.