Introduction: Nanoparticles (NP) may be used as vehicles for the intracellular delivery of biomolecules and ultimately to modulate the activity of cancer cells[1]. Here we present a novel light-dissociable polymeric NP formulation that has approximately 140nm in diameter, positive net charge, and disassemble when exposed to UV light (365nm) or blue light (405nm) releasing retinoic acid (RA). These NPs can be taken up rapidly (4h) by acute myeloid leukemia (AML) cells (K562, NB4, U937, THP1 and AML stem cells). Importantly, our results show that the light activation of NPs contribute for the differentiation of these cells at levels not observed using formulations that release passively RA or by conventional chemotherapy. Also our data shows that the activation of the NP can be done in vivo at the bone marrow (BM), using exousgenous light activation in NOD/SCID mice. This study highlights the importance of drug spatial positioning and concentration to treat leukemia.
Experimental Methods: Diameter, counts and zeta potential of NPs in response to light over time were recorded by DLS. The in vitro effects of RA-loaded NPs on: (i) the erythroid differentiation of K562 at day 9 was assessed by cytochemical staining with benzidine solution, (ii) the granulocytic differentiation at day 6 and 3 for human NB4 and U937 (PLZF/RARA), respectively, was assessed by flow cytometry using CD13 and CD11b as a marker, (iii) reduction of stem cell colonies in AML stem cells was assessed by colony-forming cell (CFC, 2 weeks) and long-term culture initiating cell assays (LTC-IC, 7 weeks). The in vivo effects of RA-NP on homing, engraftment and differentiation of AML cells was performed by injecting intravenously NP-loaded THP1 cells in NOD/SCID mice. At day 6 the NP were light activated and 72h later mice were sacrificed for analysis. The effects at the calvaria BM were accessed by flow cytometry at day 6 and by ex-vivo imaging of the calvaria at day 9, using CD45 and CD11b markers. Three independent runs were done for each experimental group and for each run three technical replicates were performed.
Results and Discussion: The activation of NPs promotes rapid and efficient RA delivery. Light-activated RA-NPs induce 1.92 (± 0.17) times higher levels of erythroid differentiation in K562, 1.19 (±0.01) times of granulocytic differentiation in NB4 and 1.45 (±0.03) times of granulocytic differentiation in U937 cells as compared to non-activated NPs. In addition, activated RA-NPs induce 1.83 (±0.17) times higher levels of erythroid differentiation in K562, 1.12 (±0.01) times of granulocytic differentiation in NB4 and 1.44 (± 0.04) times of granulocytic differentiation in U937 cells as compared to 1 mM RA in solution. It should be noted that RA-NPs contain ~10 times less RA than the one used in solution. This experimental result is particularly important in the RA-low sensitive cell line U937-PLZF/RARA where the high RA intracellular release is able to overcome the low sensitivity of the cell. AML stem cells treated with light-activated RA+-NPs showed 69.6 ± 9.2 % (CFC) and 61.8 ± 10.1 % (LTC-IC) less colonies.
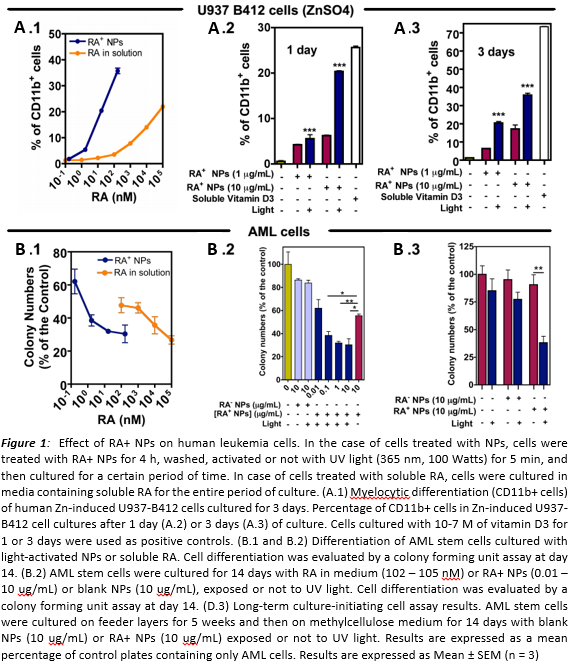
In agreement with the in vitro results, we were also able to induce differentiation of THP1 cells in the calvaria BM niche without affecting homing or engraftment. These results indicate that the enhanced intracellular release and the kinetic control mediated by the light triggered-NPs may be an effective strategy to treat leukemia.
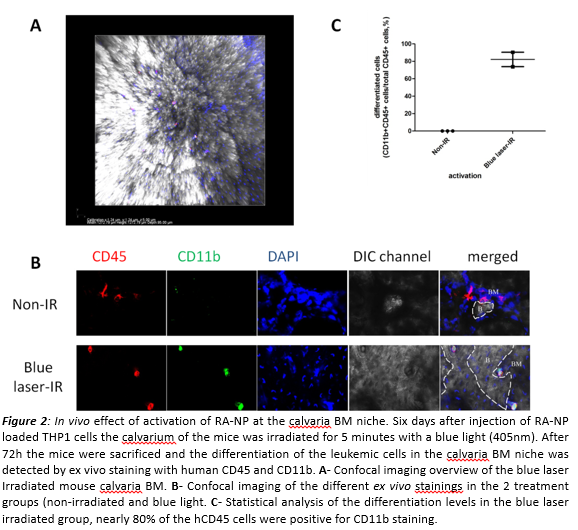
Conclusion: The spatio-temporal control of the intracellular release of RA is important to control the differentiation of leukemic (stem) cells.
Fundação para a Ciência e Tecnologia - FCT
References:
[1] Ferreira L. et al., Cell Stem Cell (2008), 3(2):136-146