INTRODUCTION
Recently, biofunctionalized magnetic nanoparticles are investigated for cancer theragnosis applications[1]. These “intelligent” particles are comprised by a magnetic core, a biocompatible surface coating, a therapeutic agent, and a recognition layer represented by a suitable receptor attached[2]. Amphiphilic derivative chitosan was used to design nanoparticles as a cancer drug delivery system with promising results regarding tumor growth inhibition[3]. Recent studies have revealed that biotin receptors are overexpressed on the surface of tumoral cells, especially breast cancer cells and imply that biotin can be used as a tumor targeting approach for various anti-cancer drugs[4]. Doxorubicin (DOX) is known as one of the most important chemotherapeutic agent against breast, ovarian or lung cancer cells. In this context, the main objective of this study was to design biofunctionalized magnetic nanostructures based on magnetite and N-palmitoyl chitosan, with dual entrapment (therapeutic agent and magnetic material) and evaluate their physico-chemical and biological characteristics.
EXPERIMENTAL METHODS
Biofunctionalized magnetic nanoparticles (BMNs) have been prepared in two steps: first, nanostructures based on N-palmitoyl chitosan, loaded with doxorubicin and hydrophobic magnetite have been obtained by a double emulsion method; in the next step, magnetic nanoparticles have been functionalized with biotin via carbodiimide chemistry. BMNs structure have been investigated and confirmed by Fourier transform infrared spectroscopy (FT-IR) and X-ray diffraction analysis. Particles size and zeta potential were measured with a Malvern Zetasizer NanoS instrument; Magnetic properties have been evaluated using a vibrating sample magnetometer (Lakeshore VSM 7400 System) and the surface morphology was studied by transmission electron microscopy (TEM; PHILIPS CM 20). Drug loading efficiency and in vitro kinetic of drug release has been assessed by UV-VIS Spectroscopy. Internalization of biofunctionalized magnetic nanoparticles was investigated by fluorescence microscopy on MCF-7 cells (Fig 1).
RESULTS AND DISCUSSION
A new type of biofunctionalized magnetic nanoparticles with submicron size, positive zeta potential, dual entrapment ability, cytotoxic effects on MCF7 cell line human breast adenocarcinoma, redispersion ability and suitable magnetic properties (good magnetic saturation and superparamagnetic behaviour) have been prepared. FT-IR confirmed the structure of biofunctionalized magnetic nanocarriers: biotin, N-palmitoyl chitosan, magnetite and doxorubicin. TEM image has confirmed that hydrophobic magnetite was effectively incorporated into the magnetic nanoparticles.
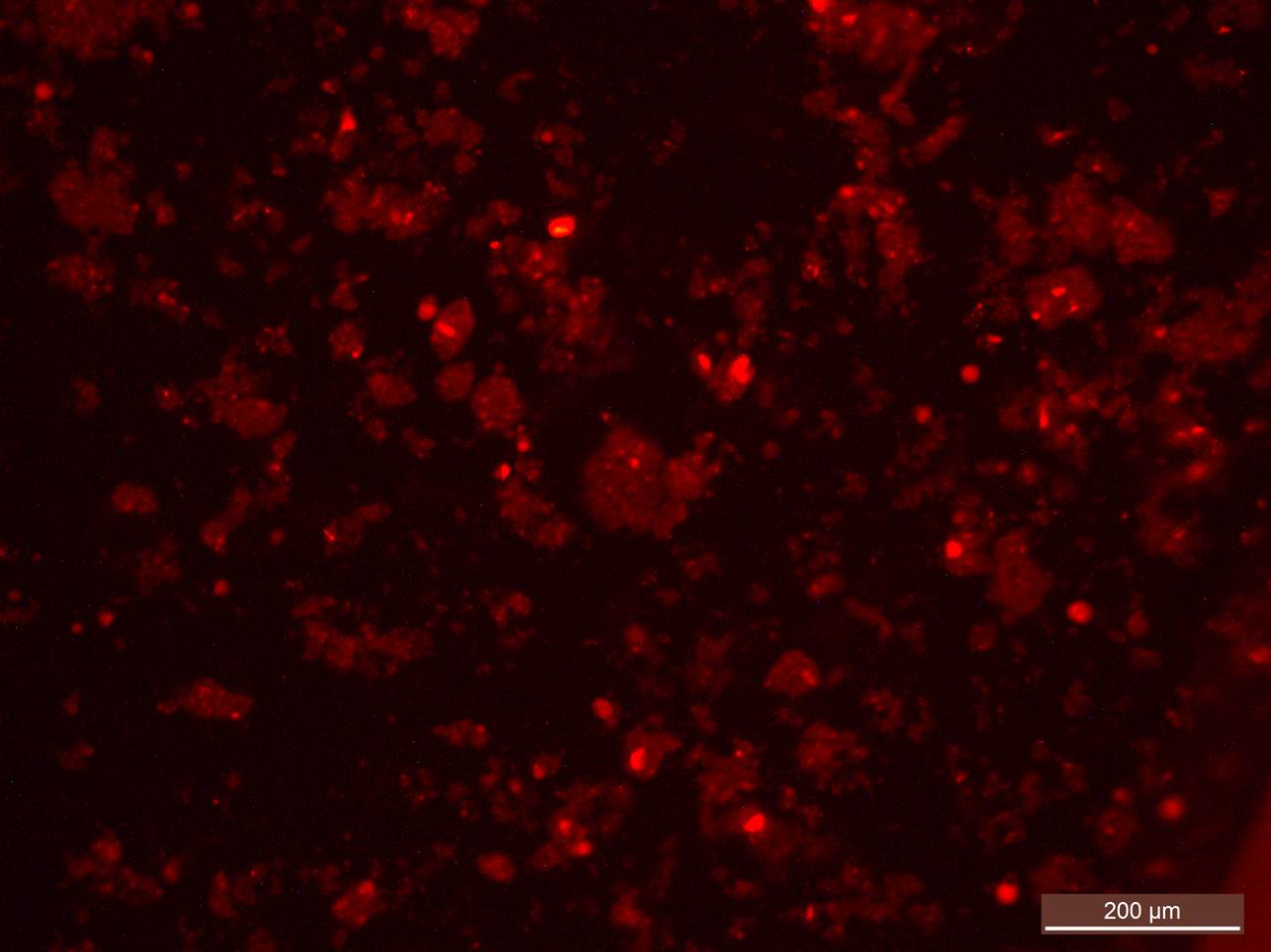
Fig. 1. The fluorescent microscopy image of BMNs in contact with MCF cells
CONCLUSIONS
These particles exhibit a multitude of good assets together with suitable biological behavior that sustain future experiments aiming to confirm the suitability of proposed magnetic nanoparticles as drug delivery system for breast cancer chemotherapy.
This work was financially supported by the Romanian Ministry of Education; grant PN II- PT-PCCA-2011-3.2-0428 - INTERBIORES.
References:
[1] Ling Y et al. Biomaterials (2011), 32:7139-7150;
[2] Chomoucka J et al. Pharmacol Res (2010), 62:144–149;
[3] Larsson et al. Prog. Polym. Sci. (2013) 38: 1307-1328;
[4] Zhu W. et al. J. Colloid Interface Sci. (2015) 443: 1–7.