Introduction: In the present work the synthesis of 3D hybrid porous scaffolds was performed through a freeze drying method, aiming at the development of implantable materials with enhanced biocompatibility and bioactivity. These hybrid materials constitute an innovative alternative for implant development, in order to successfully replace damaged human tissue and provide adequate mechanical stability till the natural regeneration of the desired tissue. The hybrid scaffolds consist of hydroxyapatite nanocrystals, synthesized biomimetically in the presence of natural biomolecules, in an effort to tailor the nucleation process and control the hydroxyapatite’s crystal growth. Since these scaffolds serve as a template to support and guide formation of new tissue, the material is required to present a porous network with high interconnectivity and mechanical stability[1],[2]. However, the porous structure of the scaffolds, inevitably affects the material’s mechanical properties. For this purpose, chemical cross-linking was performed with organic crosslinkers, through bonding with the biomolecules’ amino groups[3],[4].
Materials and Methods: Aqueous suspensions of hybrid nanohydroxyapatite were prepared through a bioinspired approach[5],[6] and then they were undertaken lyophilization to develop porous 3D scaffolds. To improve scaffolds’ mechanical properties, cross-linking of the biomolecules was performed prior to lyophilization. Finally, the physicochemical and biological properties of the as-obtained scaffolds were examined.
Results: Evaluation of scaffolds’ internal porosity with Scanning Electron Microscopy (SEM) and X-ray computed microtomography (micro-CT) indicates the development of a highly interconnected porous network with bimodal pore size distribution (Fig. 1).
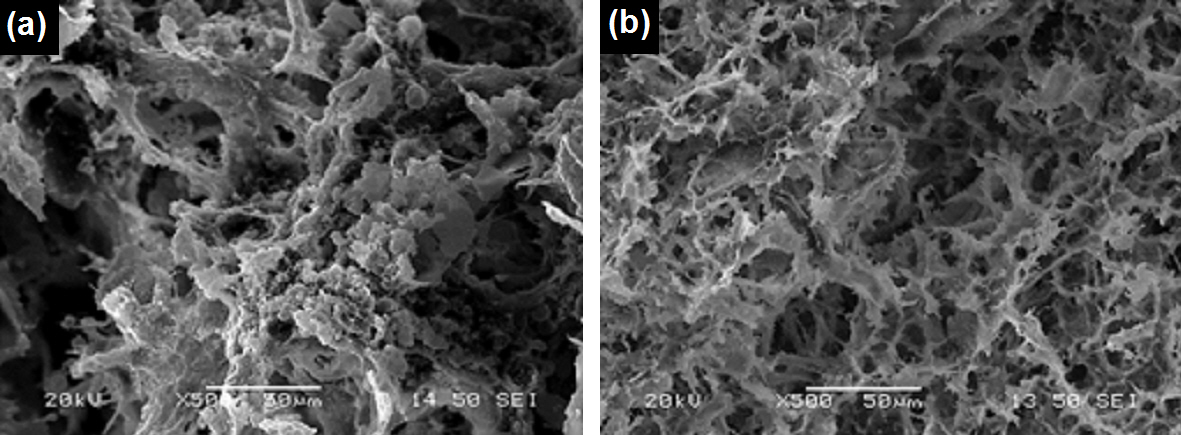
Fig. 1: SEM images of the internal structure of hybrid scaffolds differing in the biomolecules employed for increasing mechanical stability.
Optimization work provided scaffolds with pore size ranging from 50 to 100 μm for the large pores and around 5 to 10 μm for the smaller ones. Cell viability and cytotoxicity tests (MTT assay and fluorescein diacetate/propidium iodide staining (FDA/PI)) proved that after 7 days of cultivation a well-populated biomaterial was obtained with viable cells distributed through the scaffold (Fig. 2).
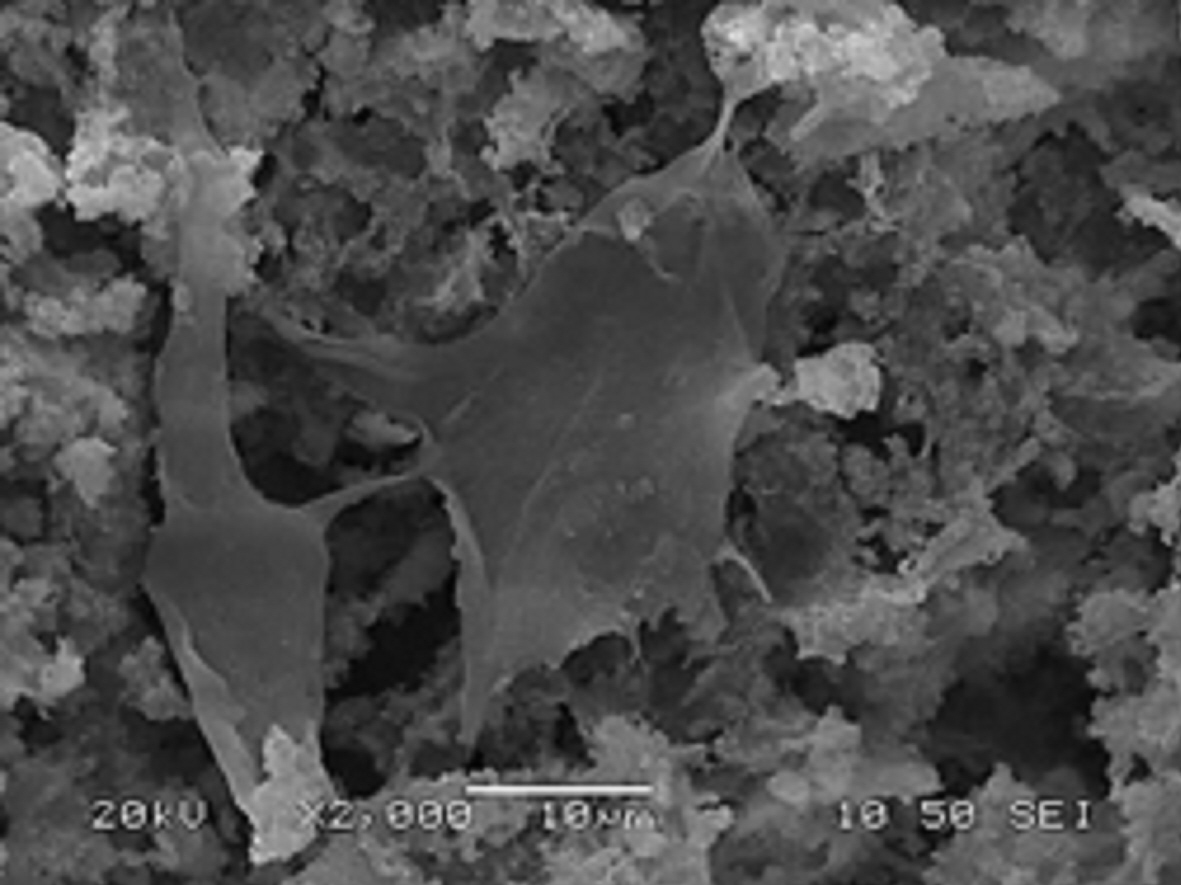
Fig. 2: SEM image of osteoblasts adhered into the porous structure of a 3D scaffold after 7 days of cultivation.
Discussion: The adopted bioinspired approach led to the fabrication of 3D hybrid porous scaffolds with a complex architecture and high interconnectivity among the pores. Their chemical composition and nanostructure - resembling that of natural bone - along with their complex porous network provide a cell friendly environment which proved beneficial for cell distribution, attachment and proliferation.
Conclusions: The as-produced hybrid scaffolds comprise biocompatible biomaterials with promising properties for tissue engineering applications.
The research leading to these results has received funding from National Strategic Reference Framework 2007–2013, Cooperation programs, Project BIOMINY – 09SYN-41-757
References:
[1] P. Habibovic, H. Yuan, C.M. van der Valk, G. Meijerc, C.A. van Blitterswijk, K. de Groot. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005;26:3565–3575.
[2] R.D.A. Gaasbeek, H.G. Toonen, R.J. van Heerwaarden, P. Buma. Mechanism of bone incorporation of β-TCP bone substitute in open wedge tibial osteotomy in patients. Biomaterials 2005;26:6713–6719.
[3] L.P. Yan, Y.J. Wang, L. Ren, G. Wu, S.G. Caridade, J.B. Fan, L.Y. Wang, P.H. Ji, J.M. Oliveira, J.T. Oliveira, J.F. Mano, R.L. Reis. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010;95:465–475.
[4] L. Bi, Z. Cao, Y. Hu, Y. Song, L. Yu, B. Yang, J. Mu, Z. Huang, Y. Han. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J. Mater. Sci.: Mater. Med. 2011;62:22-51.
[5] A. Tsetsekou, D. Brasinika, V. Vaou, E. Chatzitheorides. On the synthesis of tailored biomimetic hydroxyapatite nanoplates through a bioinspired approach in the presence of collagen or chitosan and L-arginine. Mater. Sci. Eng. C. 2014;43:555-565.
[6] D. Brasinika, O. Tsigkou, A. Tsetsekou, Y.F. Missirlis. Bioinspired synthesis of hydroxyapatite nanocrystals in the presence of collagen and l-arginine: candidates for bone regeneration. J. Biomed. Mater. Res. B: Appl. Biomater. 2015 (in press).