Background: The Department of Plastic Surgery in our hospital frequently performs bone grafts in general practice. For bone grafting, the patient’s own bone needs to be sampled invasively. If such an invasive operation can be avoided through the application of regenerative medicine, this will greatly benefit both surgeons and patients. Therefore, in our department, we perceived the cleft lip and palate for which surgery would be required several times. We culture and cryopreserve mesenchymal cells from the osseous tissue which became a surplus of the first surgery. In this congress in Denver 2014, we introduced the probability that the cryopreserved autologous mesenchymal cells derived from bone(hBMCS) would become a regenerative bone therapy.[1] This time, for the purpose of enhancing bone regeneration, we investigate the availability of conditioned medium(CM) from human autologous bone-derived mesenchymal cells.[2]
Methods:
[ Cell preparation] We used hBMCs that had undergone long-term cryopreservation at −80°C. Six samples were obtained from6male patients aged 5–7 years (mean age: 6.2 years). No subjects had any infections or diseases of note. We recultured hBMCs in vitro following cryopreservation for ≥10 years, the cells were into osteogenic nondifferentiation.
[Preparation of conditioned medium] hBMSCs that were 70%–80% confluent were re-fed with serum-free αMEM containing antibiotic-antimycotic. The cell-cultured conditioned media were collected after a 48-hr incubation. The collected, cultured conditioned medium (CM) were freeze-dried. and were stored at 4°C before being used for the following experiments. We prepared regular CM and ten times concentrated CM.
[Enzyme-linked immunosorbent assay analyses] The levels of IGF-1, VEGF, HGF in hBMCs –CM were investigated using enzyme-linked immunosorbent assay (ELISA).
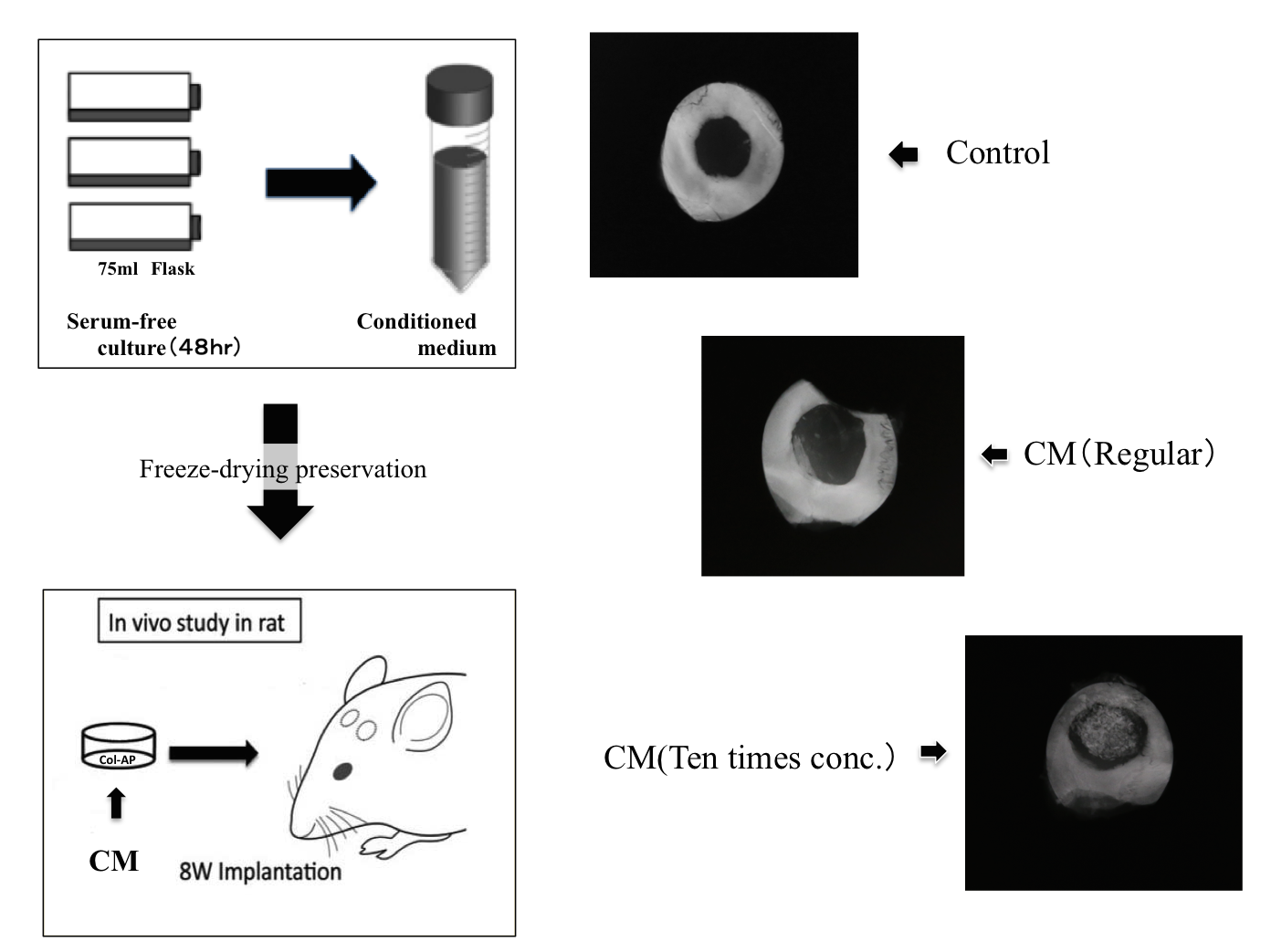
[In vivo study] we created a apatite-collagen (Rifit ®) + hBMCs -CM bone substitute and transplanted it into 5-mm diameter holes in the left and right sides of the skulls of 8-week-old male nude rats. Eight weeks later, the skulls were examined by X-ray findings. We then performed microscopic analysis of grafts after hematoxylin and eosin (HE) staining.
Results: X-ray finding of the calvaria 8 weeks after implantation :In the PBS, hBMCs-CM(Regular), the defect was left unfilled, while in the hBMCs-CM(Ten times conc.) the defect was filled with new bone(Fig.1).
The concentration of the growth factors IGF-1, VEGF, HGF, released by hBMCs into CM, was quantified using ELISA analysis. Growth factors were not detected in IGF-1. However, hBMCs-CM contained VEGF and HGF, Especially VEGF was a higher level (Table 1).
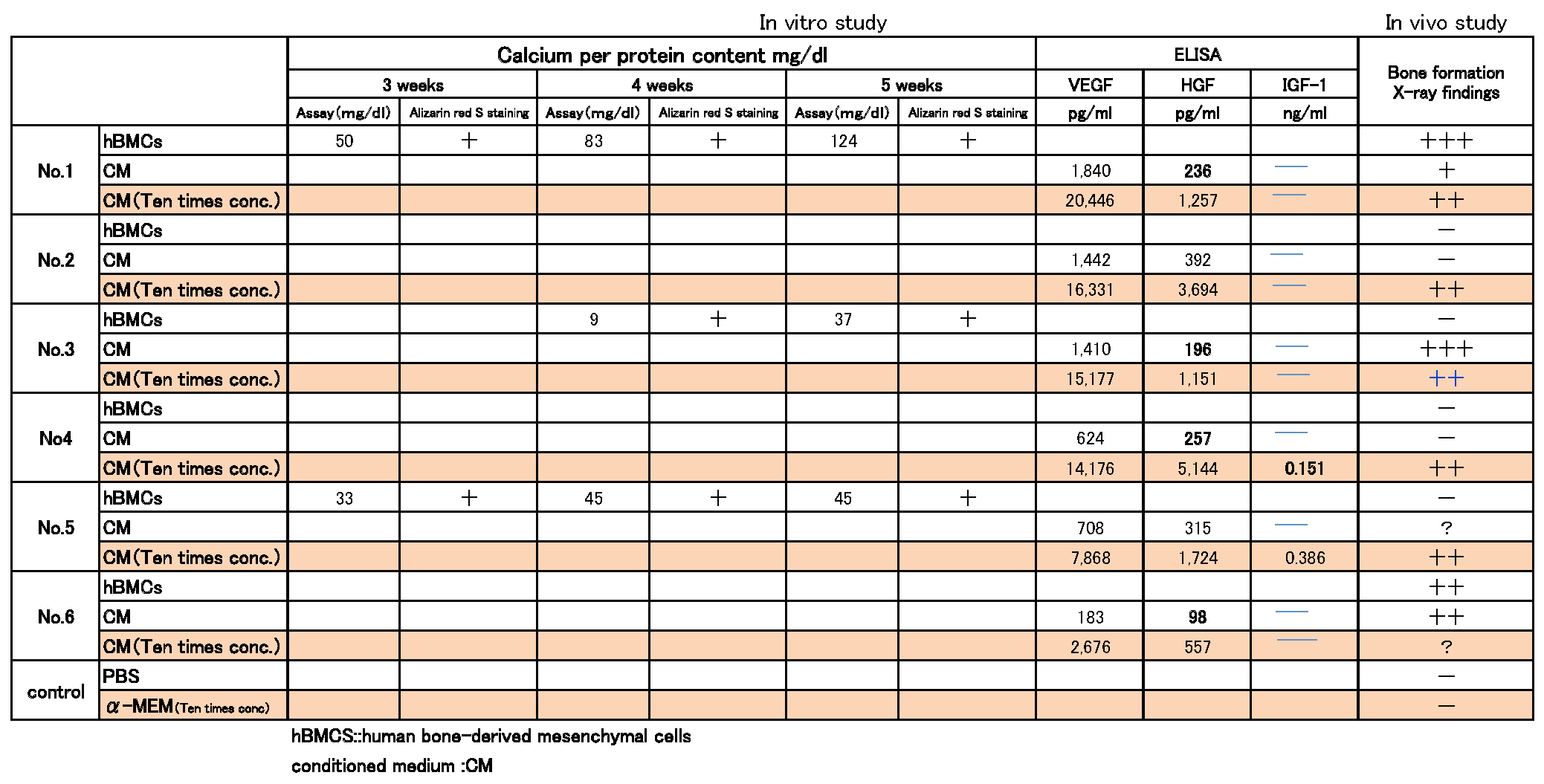
Conclusions: This investigation indicated that hBMCs-CM would enhance bone regeneration.
References:
[1] Sugimoto T, Yamazaki Y et al: The Significance of Performing Osteogenic Differentiation in Human Bone Tissue-Derived Mesenchymal Stromal Cells. Journal of oral tissue engineering 11:103-112,2013
[2] Osugi M, et al: Conditioned Media from Mesenchymal Stem Cells Enhanced Bone Regeneration in Rat Calvarial Bone Defects. TISSUE ENGINEERING: Part A 18:1481-1489, 2012.