Adipose derived stromal cells encapsulation in hydrogel particles: potential application to osteoarthritis
Fahd
Hached1, 2,
Claire
Vinatier1, 2, 3,
Pierre-Gabriel
Pinta1, 2, 3,
Pierre
Weiss1, 2, 3,
Catherine
Le Visage1, 2,
Philippe
Hulin4,
Aurélie
Billon-Chabaud1, 2, 5,
Jérôme
Guicheux1, 2, 3 and
Gaël
Grimandi1, 2, 3, 5
-
1
INSERM U791, LIOAD, France
-
2
University of Nantes, Faculty of Dentistry, France
-
3
Ministry of Health, Nantes University Hospital, France
-
4
INSERM UMS 016, SFR François Bonamy / MicroPicell platform, France
-
5
University of Nantes, Faculty of pharmacy, France
Introduction
Osteoarthritis (OA) is a degenerative and inflammatory joint disease that affects cartilage, subchondral bone and joint tissues[1]. Mesenchymal Stromal Cells (MSCs) have generated interest since they secrete immuno-modulatory and anti-inflammatory factors[2]. Intra-articular injection of MSCs suffers major limitations including a cell death upon injection[3] and a massive leakeage outside the articular space[4]. In this study, we proposed to entrap MSCs within particles derived from alginate or silylated hydroxypropyl methylcellulose (Si-HPMC) hydrogels.
Materials and Methods
MSCs were isolated from human adipose tissue. We used a dropwise method in CaCl2 solution and developed a water/oil emulsion protocol to obtain alginate and Si-HPMC particles, respectively. To assess the hydrogel network, particles were incubated in FITC-dextran (Mw 20 to 2000kDa) solutions for 2 hours. Diffusion profiles were assessed by confocal microscopy. Ratio of internal to external fluorescence was calculated (a ratio of 1 indicates that equilibrium is reached). Mechanical properties of the particles were investigated by subjecting them to a 30% compressive displacement for 30s (MicroSquisher). A suspension of 2.106 MSCs/ml was added either to the alginate or Si-HPMC solution, the particles were collected and cultured for up to 2 months. Cell viability was followed using a Live/Dead assay. To assess their anti-inflammatory properties, MSCs were treated for 72 hours with pro-inflammatory molecules (TNF-α, IFN-y) in the culture medium. Release of prostaglandin E2 (PGE2) was measured using a EIA Kit (Cayman) and indoleamine 2,3-dioxygenase (IDO) activity was measured by tryptophan-to-kynurenine conversion.
Results and Discussion
We obtained alginate and Si-HPMC particles with an average size of 1.5 ± 0.2 mm and 75 ± 28 µm, respectively. We evidenced a faster diffusion in Si-HPMC particles than in alginate ones
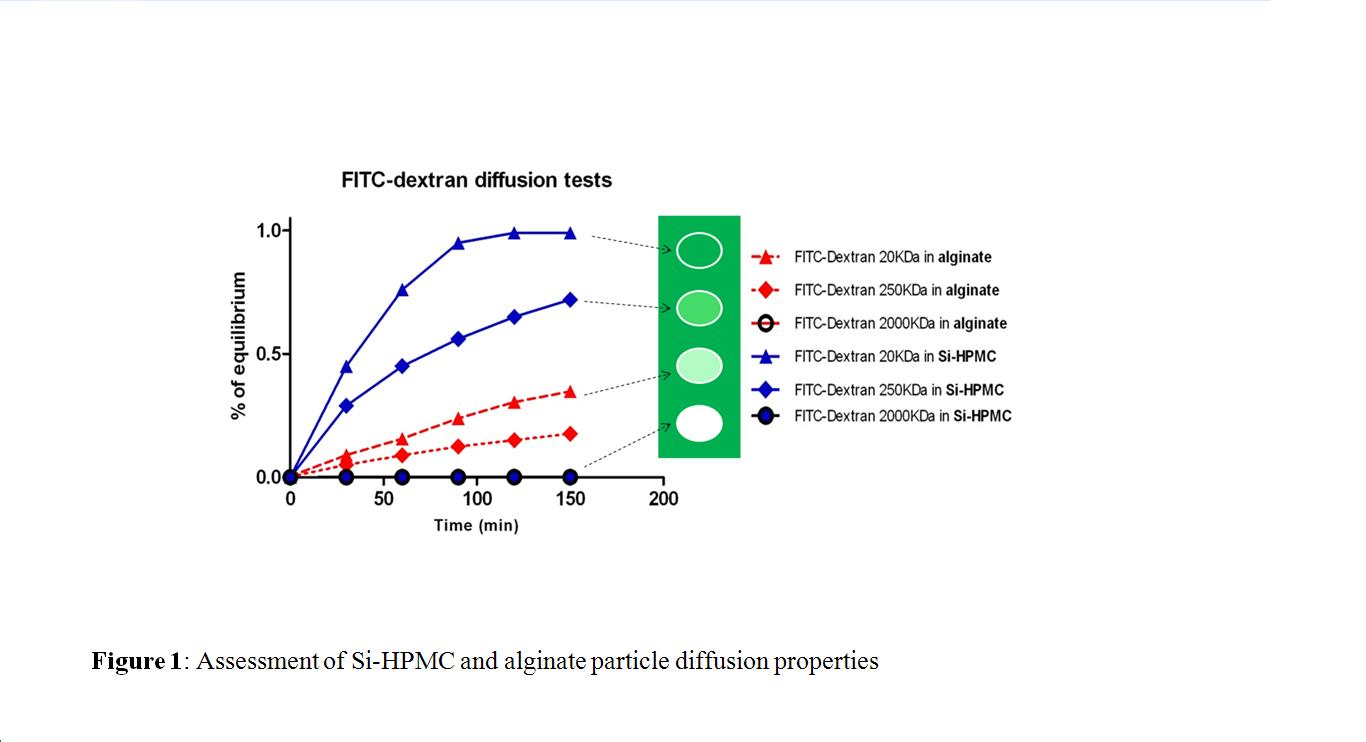
. Equilibrium was reached after 2 hours for 20 kDa dextran in Si-HPMC, with less than 30% for alginate. This data suggests that Si-HPMC could not only favor cell stimulation by the inflammatory signals but also support the release of secreted molecules. Under compression, alginate particles exhibited a Young's modulus that was 70 times greater than Si-HPMC ones 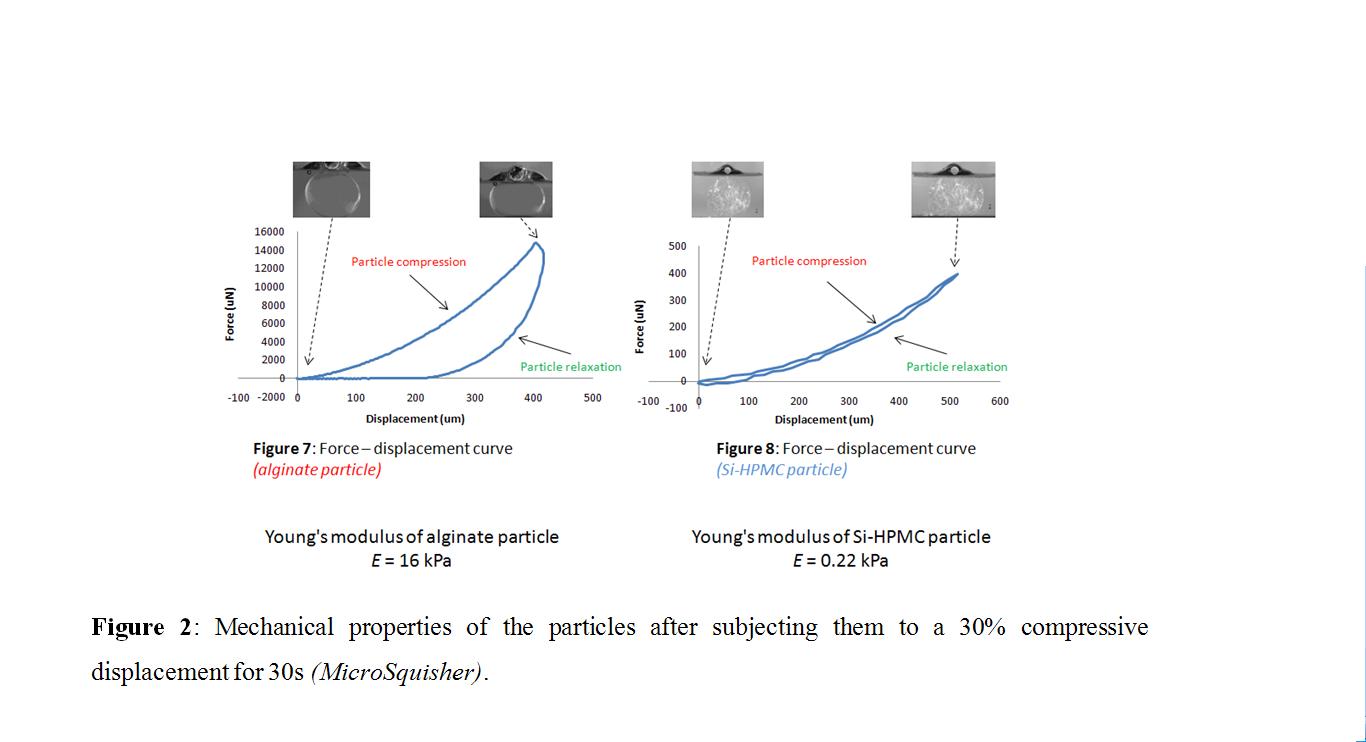
, thereby suggesting that viscoelastic Si-HPMC particles could be more effective at protecting cells from shear stress during injection and in the articular cavity. Both Si-HPMC and alginate particles supported cell survival (viability at 2 months of 93% and 90%, respectively; 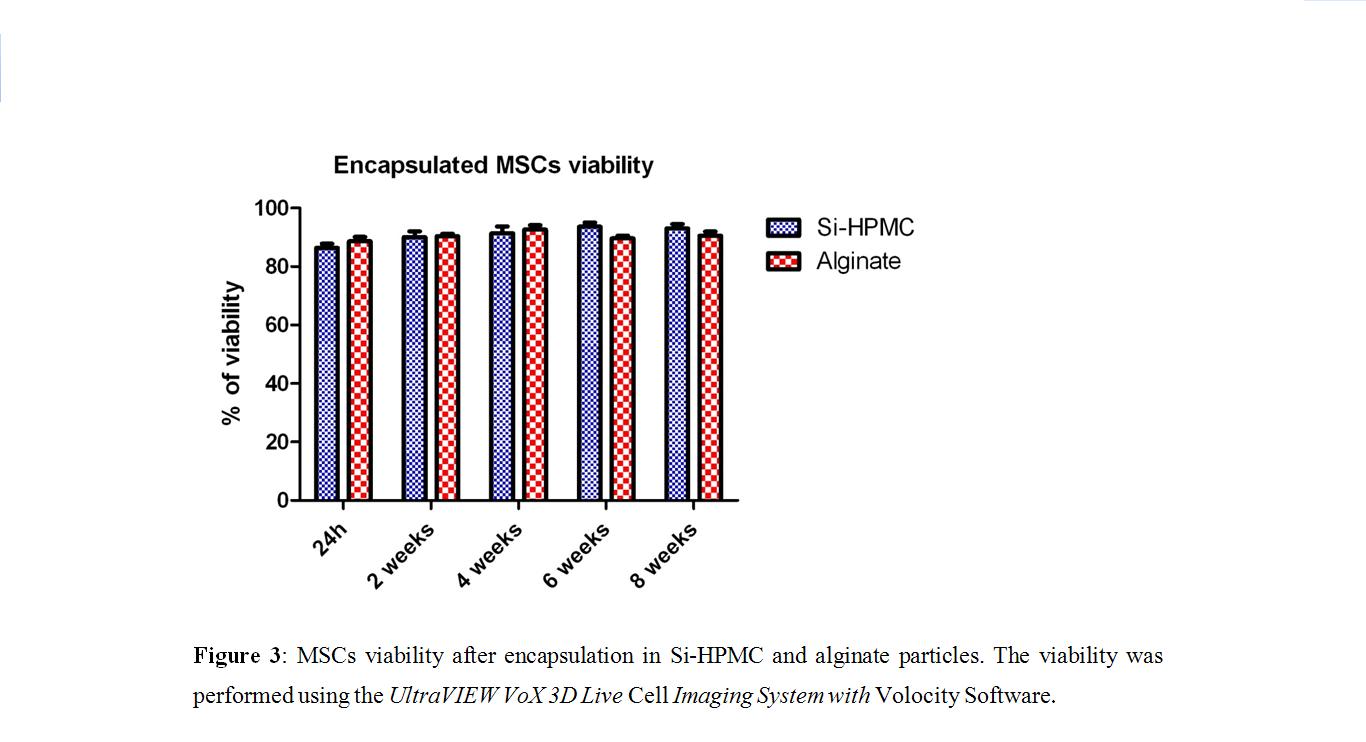
). The stimulation of encapsulated MSCs resulted in a 3-fold increase in PGE2 production in the supernatant after stimulation with pro-inflammatory cytokines, while IDO activity increased 33 times as compared to non-stimulated cells.
Conclusion
We demonstrated that Si-HPMC and alginate particles support MSCs viability and the maintenance of their anti-inflammatory properties. Diffusion assays and mechanical properties experiments indicate that Si-HPMC is a better candidate for MSCs encapsulation than alginate. Future experiments will investigate whether encapsulated MSCs in Si-HPMC may be a revelant strategy to prevent cartilage degradation and inflammation in OA.
Clinique Brétéché; French Society of Rheumatology; ROAD project: Research on OsteoArthritis Diseases; LMA project: Longévité Mobilité Autonomie
References:
[1] Hunter DJ (2011) Pharmacologie therapy for osteoarthritis the era of disease modification. Nature reviews Rheumatology 7: 13-22.
[2] Caplan Al, Correa D (2011) The MSC: an injury drugstore. Cell stem cell 9: 11-15.
[3] ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, et al. (2012) Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in expérimental osteoarthritis. Arthritis and rheumatism 64: 3604-3613.
[4] Hunt NC, Grover LM (2010) Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnology letters 32: 733-742.
Keywords:
stem cell,
cytokine,
Drug delivery,
Capsule
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials for therapeutic delivery
Citation:
Hached
F,
Vinatier
C,
Pinta
P,
Weiss
P,
Le Visage
C,
Hulin
P,
Billon-Chabaud
A,
Guicheux
J and
Grimandi
G
(2016). Adipose derived stromal cells encapsulation in hydrogel particles: potential application to osteoarthritis.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.00418
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.