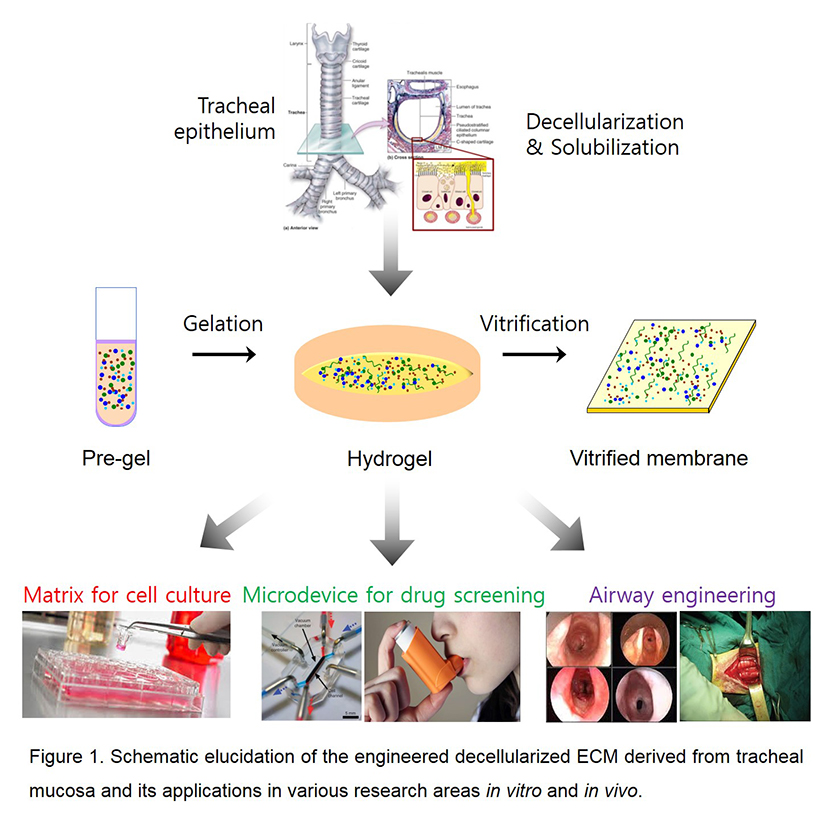
Introduction: The role of airway epithelium is to act as a primary barrier against environment agents through complex defense mechanisms which are mainly mediated by cell-cell and cell-extracellular matrix (ECM) interactions[1]. In this regard, the complete regeneration of the functional airway epithelium has been considered as a time-consuming problem due to the complex biological phenomenon[2]. Therefore, a rational strategy to facilitate functional regeneration of airway is required. This study demonstrated that a new biomaterial that mimicked natural ECM of the airway was effective for facilitating a regeneration of functional airway epithelium. Moreover, the effectiveness of this material was also confirmed in vitro and in vivo for applications in various research area (Fig. 1).
Materials and Methods: Development of decellularized tracheal mucosa-derived ECM (tmdECM): Porcine tracheal mucosa was obtained from the butcher shop and treated with 1% SDS and TritonX-100 to remove all the cellular components as previously described[3].
Human tracheal epithelial cells (HTEpCs) culture: Primary HTEpCs were purchased from ATCC. HTEpCs were grown in serum-free tracheal epithelial basal medium with growth supplements. In air-liquid interface culture of HTEpCs, cells were seeded on the transwell insert which was coated or gelled with collagen type 1 (Col-1) or tmdECM.
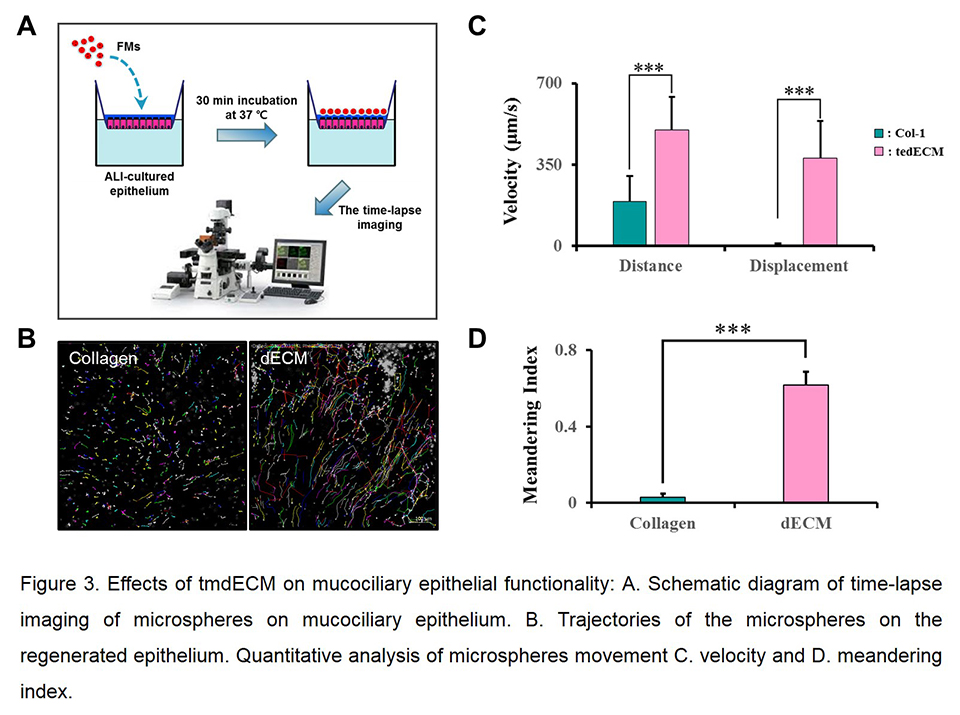
Quantitation of cilia-generated flow by videomicroscopy: Trajectories of the microspheres on the differentiated tracheal epithelium over time was analyzed by ImageJ program, and motility parameters were consequently calculated. The meandering index was calculated as the ratio of displacement to cumulative distance of microspheres.
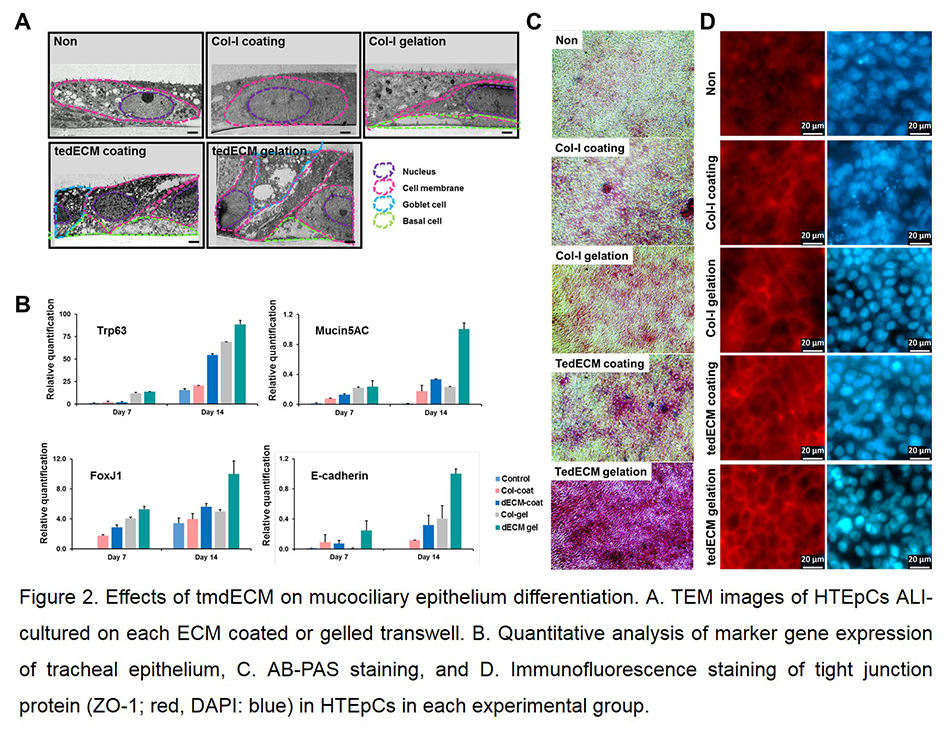
Results and Discussion: Pseudostratified columnar epithelial structure was rapidly established on tmdECM (Fig. 2A). TmdECM enhanced mucociliary epithelium differentiation by promoting expression of transcription factors (e.g. Trp63, FoxJ1) and marker genes (e.g. mucin5AC, E-cadherin) of tracheal epithelium (Fig. 2B). Functional epithelial markers including mucus secretion and tight junction expression were also highly upregulated in tmdECM group compared to Col-I (Fig. 2C and D). TmdECM hydrogel exhibited fully differentiated mucociliary epithelium which has mucociliary clearance functionality, including the velocity and meandering index, similar to native trachea (Fig. 3). To confirm the effectiveness of tmdECM for a range of applications in the field of tissue engineering and regenerative medicine, tmdECM was applied to fabricate a three-layered airway epithelium model in vitro, and formation of a directional mucus flow was observed in the model. To investigate the therapeutic efficacy of tmdECM on the airway epithelium regeneration in vivo, tmdECM printed structure was implanted in the rat tracheal defect model. TmdECM promoted tracheal epithelium regeneration by enhancing the migration of keratin-14 positive basal cells, which are mainly involved in the regeneration of defected epithelium and the formation of blood vessels.
Conclusion: TmdECM which retained all the ECM components of tracheal mucosa can be a potent biomaterial to recapitulate functional tracheal epithelium in vitro and in vivo.
This work was supported by the National Research Foundation (NRF) of Korea grant funded by the Korea government (MSIP) (No. 2010-0018294).
References:
[1] C. Coraux, J. Roux, T. Jolly, and P. Birembaut, “Epithelial cell-extracellular matrix interactions and stem cells in airway epithelial regeneration,” Proc Am Thorac Soc. Vol. 20, Aug. 2008.
[2] B. Yahaya. “Understanding Cellular Mechanisms Underlying Airway Epithelial Repair: Selecting the Most Appropriate Animal Models,” ScientificWorldJournal. 961684, Sep. 2012.
[3] F. Pati, J. Jang, D. H. Ha, S. W. Kim, J. W. Rhie, J. H. Shim, D. H. Kim and D. W. Cho, “Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink,” Nat Commun. Vol. 5, Jun. 2014.