Introduction: Regeneration of skin, containing functional appendages is a major challenge following severe skin injury such as burn. Skin derived precursors (SKPs) have the capacity to generate new dermal cells and initiate formation of hair follicles[1]. We hypothesize that autologous human SKP-loaded hydrogels with specifically engineered physico-chemical properties and spatially-arranged biological cues could provide a platform for the clinically effective healing of skin wounds. Chondroitin sulfate (CS), due to its involvement in regulation of differentiation and maintenance of stem cells as well as its capacity to bind to various regulatory proteins, is a promising material for engineering such constructs[2],[3]. Here, we describe the formation of a novel enzymatically cross-linked hybrid hydrogel consisting of CS and poly(ethylene glycol) (PEG) for establishment of skin constructs based on hSKPs.
Methods: CS was functionalized with a matrix metalloproteinase (MPP) degradable lysine donor substrate, Lys-MMP-peptide and 8-arm PEG-VS (PEG-vinyl sulfone) with FXIII recognizable peptide (NQEQVSPL). Hydrogels were formed by mixing the gel precursors in the presence of FXIII under physiologic buffer conditions. Swelling behavior, storage moduli and fluorescence recovery after photobleaching (FRAP) of hydrogels were done to characterize the network architecture. hSKPs (single cells vs. spheres) were encapsulated in PEG-CS hydrogels and cell expansion was compared by culturing the SKPs in a bioreactor. Migration of hSKPs and spheres within 3D hydrogels was studied via time-lapse confocal microscopy.
Results: A modularly assembled, TG-PEG and MMP-Lys-peptide grafted CS precursor based hybrid hydrogel was developed. Evaluation of hydrogel properties such as swelling behavior, storage moduli and diffusion coefficients of encapsulated tagged molecules, were found to be dependent on the degree of CS grafting with MMP-Lys as well as concentrations and stoichiometric ratios between the hydrogel precursors. We show that hSKPs remain viable and proliferate upon encapsulation into CS-PEG hybrid hydrogels (Fig. 1). Matrix stiffness, crosslink density and degree of swelling also influence cell viability, proliferation and migration. Further, single cells began to emigrate from encapsulated colonies after 24 hrs, suggesting that the material provided a permissive growth environment. Polar plots of overlaid cell tracks indicated that hSKPs migration occured without directional bias.
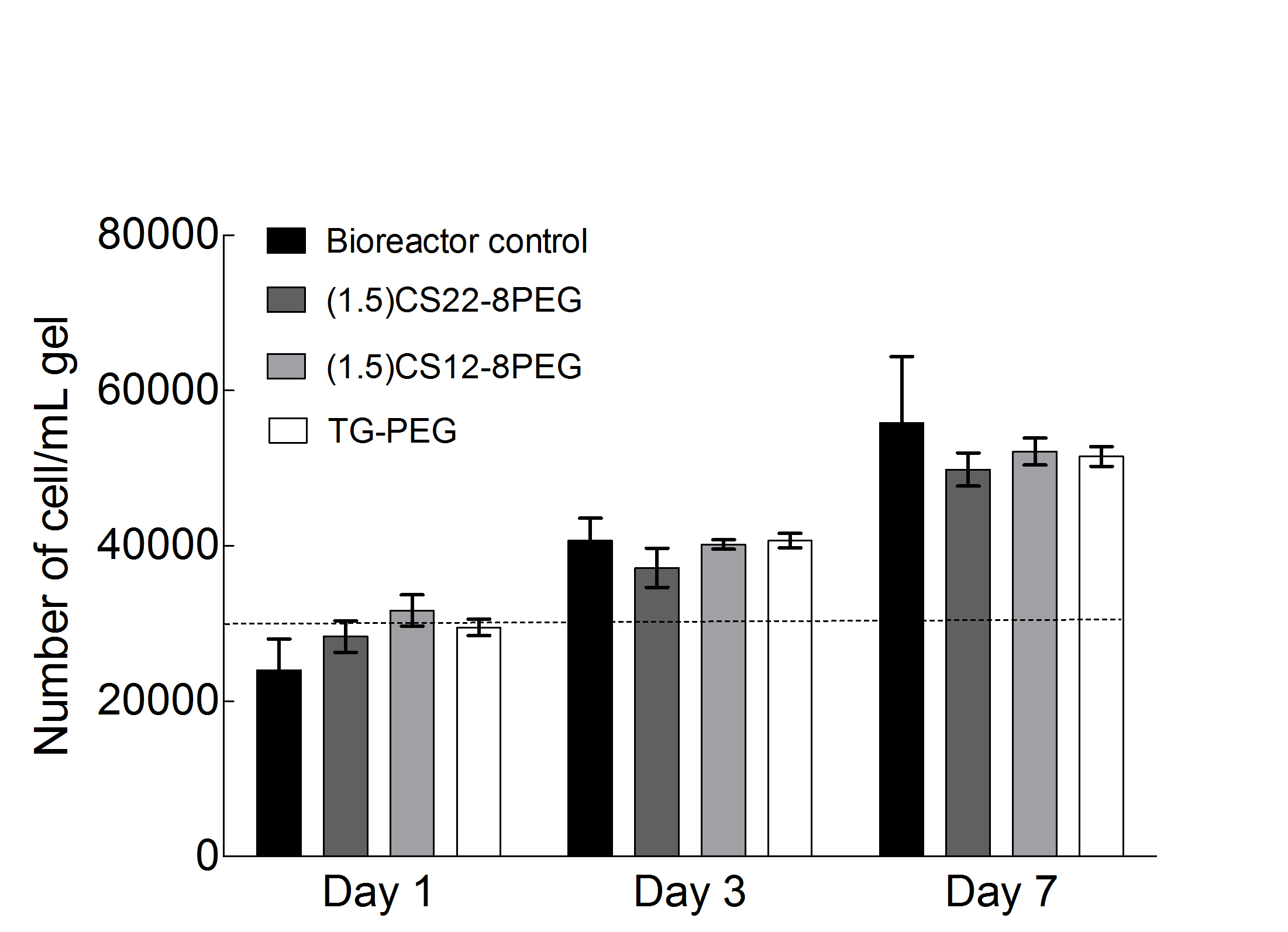
Fig. 1: SKPs cell proliferation determined by trypan blue and hemocytometer encapsulated in hybrid hydrogels. The initial cell density at day 0 was 30000 cells/gel indicated by dotted line
Conclusions: Modular designed CS-PEG hydrogels that incorporate RGD peptides and MMP degradable sequences provide a suitable substrate for hSKP proliferation and migration. Together with the ability of these substrates to bind growth factors, these scaffolds hold great promise for the engineering of functional dermis and appendages.
References:
[1] Biernaskie et al Cell Stem Cell, 2009
[2] Varghese S et al, Matrix Biol, 2008
[3] Strehin I et al, Biomaterials, 2010